Categories
Most newborns will have some jaundice. Most jaundice is benign.
So, how can we sort through the various presentations and keep our newborns safe?
Pathologic Jaundice
When a baby is born with jaundice, it’s always bad. This is pathologic jaundice, and it’s almost always caught before the baby goes home. Think about ABO-incompatbility, G6PD deficiency, Crigler-Najjar, metabolic disturbances, and infections to name a few. Newborns are typically screened and managed.
Physiologic Jaundice
Physiologic jaundice, on the other hand, is usually fine, until it’s not.
All babies have some inclination to develop jaundice. Their livers are immature. They may get a little dehydrated, especially if mother’s milk is late to come in. In today’s practice, we are challenged to catch those at risk for developing complications from rising bilirubin levels.
Hyperbilirubinemia is the result of at least one of three processes: you make too much, you don’t process it enough, or you don’t get rid of it fast enough.
Increased production
Bilirubin mostly comes from the recycling of red blood cells. Heme is broken down in in the liver and spleen to biliverdin then bilirubin.
Normal, full term babies without jaundice run a little high -- bilirubin production is two to three times higher than in adults, because they are born with a higher hematocrit. Also, fetal hemoglobin is great at holding on to oxygen, but has a shorter life span, and high turn-over rate, producing more bilirubin.
Impaired conjugation
Think of bilirubin as your email. Unconjugated bilirubin is your unread email. To process it or get rid of it – you have to open it. Of course, the more unread messages that accumulate, the more unwell you feel.
Conjugated bilirubin is your opened and processed email. So much easier to sort out, deal with, and get rid of.
Decreased excretion
Both unread email and unconjugated bilirubin continue to float around in your inbox. Unconjugated bilirubin keeps getting reabsorbed in the intestinal mucosa through enterohepatic circulation.
Processed email and conjugated bilirubin are easier to sort out. Conjugated bilirubin is water soluble, so it goes right into the read folder in your gallbladder, and is excreted off your inbox. Later on down the line in the intestine, conjugated bilirubin can’t be reabsorbed through the intestinal mucosa. Like when you open an email and forget about it – it passes on through, out of your system.
Newborns are terrible at answering emails. There is a lot of unread unconjugated bilirubin is floating around. The liver and spleen are just not able to keep up.
Also, newborns have a double-whammy administrative load. Normally, bacteria in the gut can further break down conjugated bilirubin to urobilin and get excreted in the urine. The infant’s gut is relatively sterile, so no admin assistance there. Just to add to the workload a poor little newborn has to do – he is being sabotaged by extra beta-glucuronidase which will take his hard-earned conjugated bilirubin and unconjugate it again, then recycle it, just like email you “mark as unread”.
How Does this All Go Down?
The recommended followup is 48 hours after discharge from the nursery for a routine bilirubin check, often in clinic, and often via the transcutaneous route.
More Specifically:
| Infant Discharged | Should Be Seen by Age |
| Before age 24 h | 72 h |
| Between 24 and 48 h | 96 h |
| Between 48 and 72 h | 120 h |
The neonate will end up in your ED off hours, if there is concern, if his status deteriorates, or simply by chance. We need to know how to manage this presentation, because time is of the essence to avoid complications if hyperbilirubinemia is present.
Critical Action #1:
Assess risk for developing severe hyperbilirubinemia.
This will tell you: check now in ED or defer to clinic (default is to check).
| Risk Factors for Developing Hyperbilirubinemia |
| Total serum bilirubin/Transcutaneous bilirubin in high-risk zone |
| Jaundice in first 24 hours |
| ABO incompatibility with positive direct Coombs, known hemolytic disease, or elevated ETCO |
| Gestational age 35-36 weeks |
| Prior sibling had phototherapy |
| Cephalohematoma or bruising |
| Exclusive breastfeeding, especially with poor feeding or weight loss |
| East Asian Race |
Critical Action #2
Check bilirubin and match this with how old the child is -- in hours of life -- at the time of bilirubin measurement.
This will tell you: home or admission.
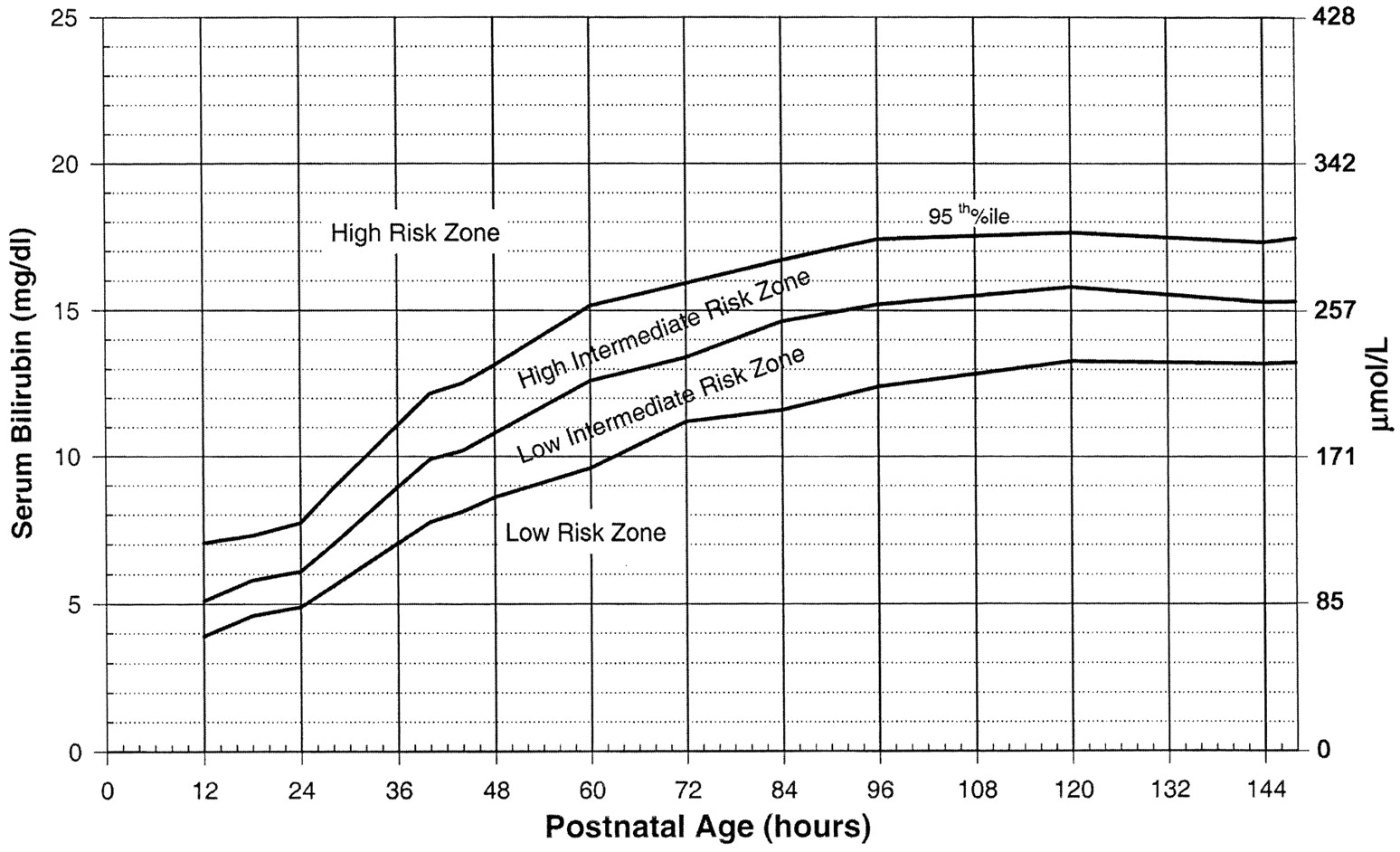
Use the Bilitool or Bhutani Nomogram (below).
Can I go Home Now?
In general, babies at low-risk and low-intermediate risk can go home (see below). Babies at high-intermediate or high risk are admitted (see below).
Critical Action #3:
Assess risk for developing subsequent neurotoxicity.
This will tell you: a) phototherapy or b) exchange transfusion
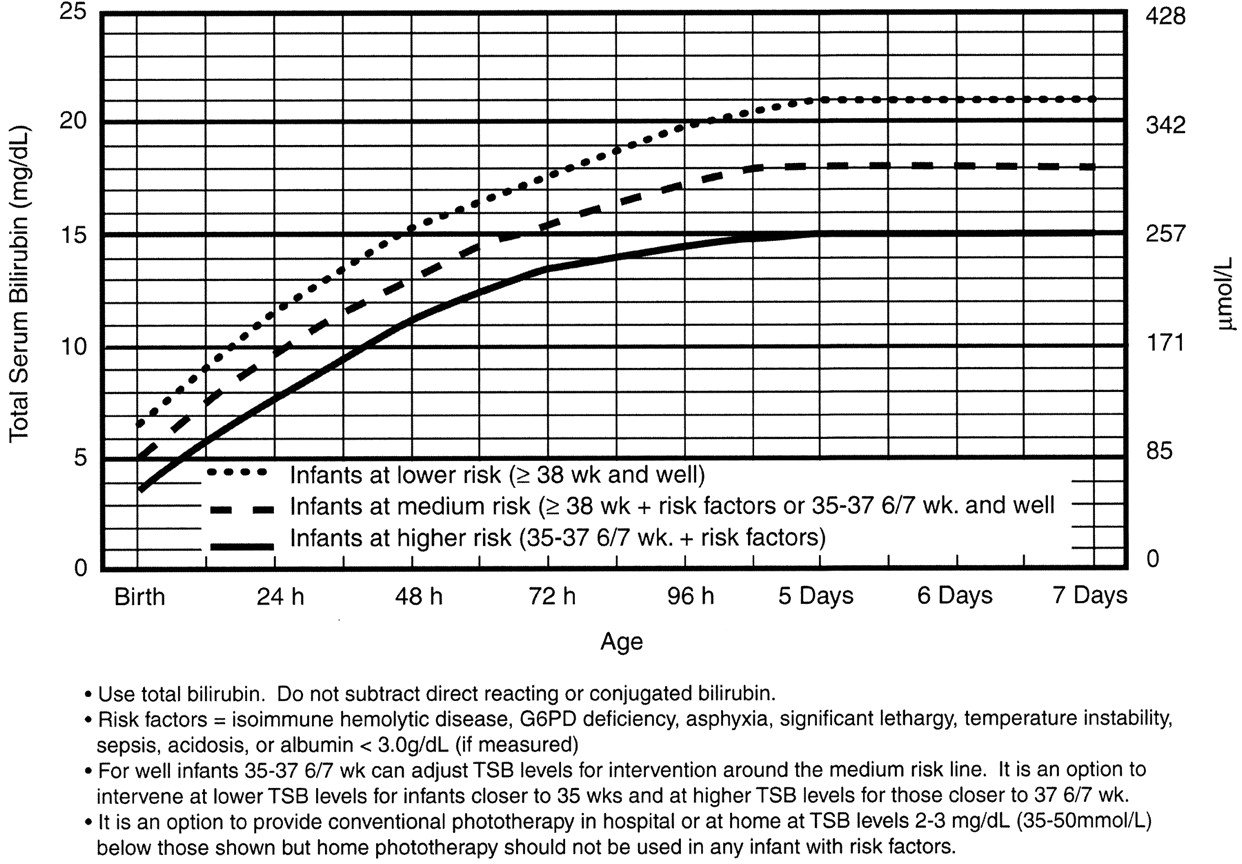
Phototherapy Now?
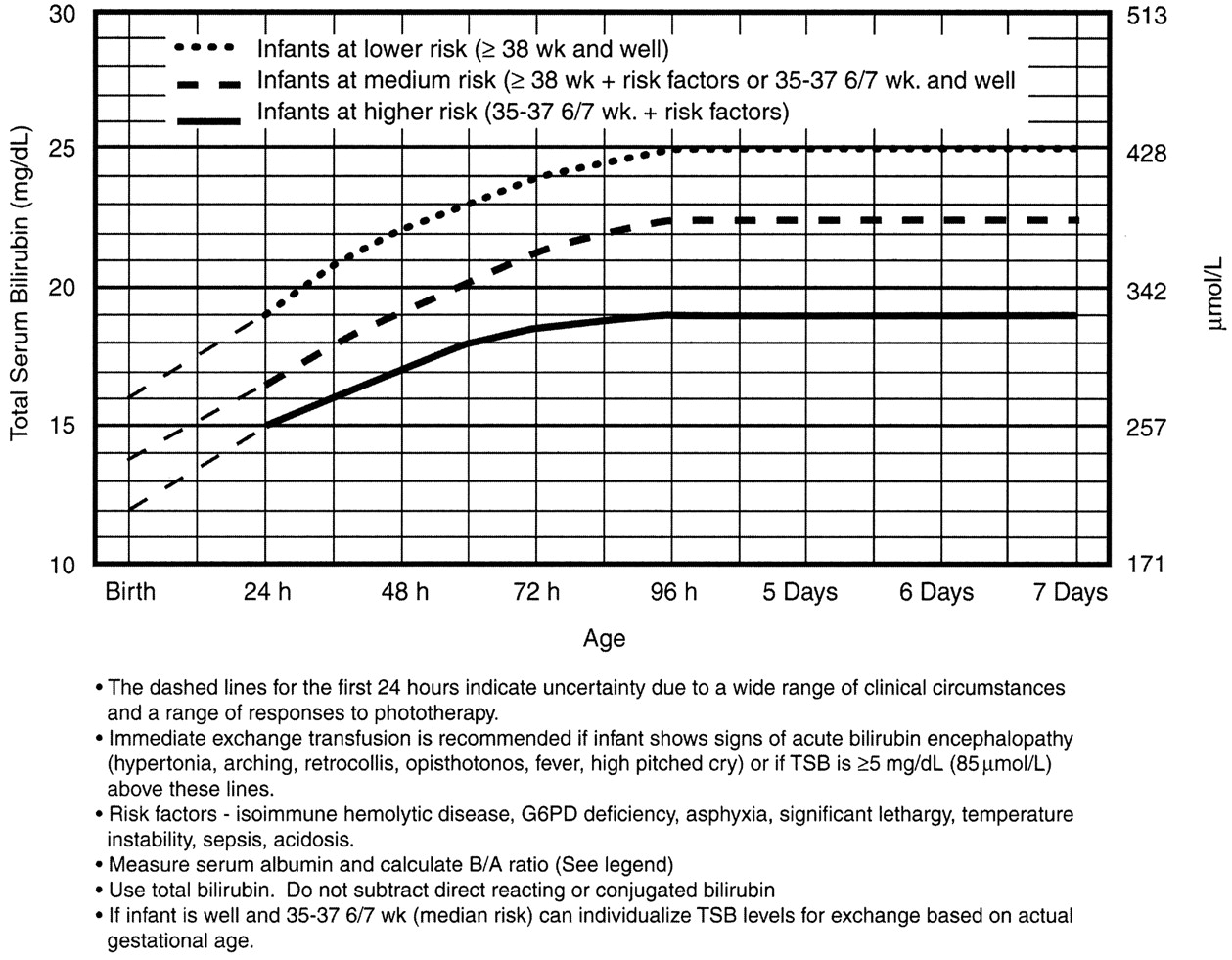
Exchange Transfusion Now?
Home care
The neonate who is safe to go home is well appearing, and not dehydrated. His total bilirubin is in the low to low-intermediate risk for developing severe hyperbilirubinemia, and he is not at high risk for neurotoxicity based on risk factors.
Babies need to stay hydrated. Breast feeding mothers need encouragement and need to offer feeds 8-12 times/day – an exhausting regimen. The main message is: stick with it. Make sure to enlist the family's help and support to keep Mom hydrated, eating well, and resting whenever she can. Supplementing with formula or expressed breast milk is not routinely needed. Be explicit that the neonate should not receive water or sugar water – it can cause dangerous hyponatremia. A moment of solid precautionary advice could avert a disaster in the making.
The child’s pediatrician will help more with this, and you can remind nursing mothers of the excellent La Leche League – an international group for breastfeeding support. They have local groups everywhere, including a hotline to call.
Nursery Care
If the baby is at high intermediate or high risk for hyperbilirubinemia, then he should be admitted for hydration, often IV. Most babies with hyperbilirubinemia are dehydrated, which just exacerbates the problem.
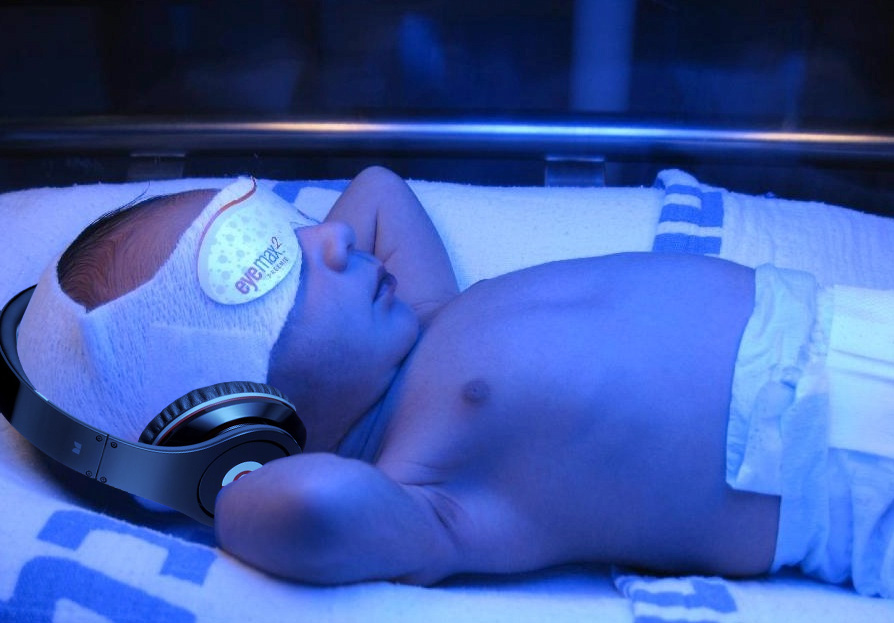
Bililights or biliblankets, provide the baby with the right blue spectrum of light to isomerize bilirubin to the more soluble form. Traditionally, we have thought them to be more effective or safer than filtered sunlight. A recent randomized control trial by Slusher et al. in the New England Journal of Medicine compared filtered sunlight versus conventional phototherapy for safety and efficacy in a resource-poor environment. These were all term babies with clinically significant jaundice in Nigeria. To standardize the intervention, they used commercial phototherapy canopies that remove most UV rays. None of them became dehydrated or became sunburned. The filtered sunlight resulted in a 93% successful treatment versus 90% for conventional phototherapy. My take away: we now have some evidence basis for using filtered sunlight as an adjunct for babies well enough to go home.
Critical Care
Although rare, the critically ill neonate with hyperbilirubinemia requires immediate intervention.
He will be dehydrated – possibly in shock. He will be irritable.
Or, he may just have a dangerously high bilirubin level – at any minute he could develop bilirubin induced neurologic dysfunction, or BIND, especially when bilirubin concentrations reach or surpass 25 mg/dL (428 micromol/L). The bilirubin is so concentrated that it leeches past the blood brain barrier and causes neuronal apoptosis. BIND is a spectrum from acute bilirubin encephalopathy to kernicterus, all involving some disorder in vision, hearing, and later gait, speech, and cognition.
Acute bilirubin encephalopathy starts subtly. The neonate may be sleepy but hypotonic or have a high-pitched cry; he maybe irritable or inconsolable, jittery or lethergic.
The dehydration and neurologic dysfnction from the hyperbilirubinemia may even cause fever. Check the bilirubin in any neonate you are working up for sepsis.
Acute bilirubin encephalopathy may progress to an abnormal neurologic exam, seizures, apnea, or coma.
Kernicterus is the final, permanent result of bilirubin encephalpathy. The child may have choreoathetoid cerebral palsy with chorea, tremor, ballismus, and dystonia. He may have sensorineural hearting loss, or cognitive dysfunction.
It is for this reason that any child sick enough to be admitted should be considered for exchange transfusion. Most babies need just a little gentle rehydration and bililights, but to be sure, the admitting team will look at a separate nomogram to gage the child’s risk and decide whether to pull the trigger on exchange transfusion. For our purposes, a ballpark estimate is that if the total serum bilirubin is 5 mg/dL above the phototherapy threshold, or if they have any red flag signs or symptoms, then exchange transfusion should be started.
Exchange transfusion involves taking small aliquots of blood from the baby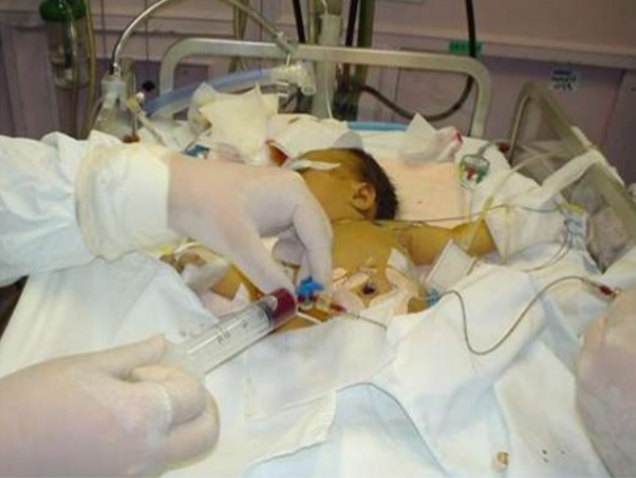 and replacing them with donor blood. It’s often a manual procedure, done with careful monitoring. It can be done with any combination of umbilical arteries or veins with peripheral arteries or veins. In general, arteries are the output, veins are for transfusion. The baby may need a double-volume exchange, which ends up replacing about 85% of circulating blood, a single-voume exchange, replacing about 60% of blood, or any fraction of that with apartial volume exchange. It is a very delicate procedure that requires multiple hours and often multiple staff.
and replacing them with donor blood. It’s often a manual procedure, done with careful monitoring. It can be done with any combination of umbilical arteries or veins with peripheral arteries or veins. In general, arteries are the output, veins are for transfusion. The baby may need a double-volume exchange, which ends up replacing about 85% of circulating blood, a single-voume exchange, replacing about 60% of blood, or any fraction of that with apartial volume exchange. It is a very delicate procedure that requires multiple hours and often multiple staff.
For our pruposes, just be aware that the jaundiced baby in front of you may need escalation of his care.
Summary
Find out the hour of life of the baby at the time of bilirubin measurement. Identify risk factors for developing severe hyperbilirubinemia and/or neurotoxicity
The child with low to low-intermediate risk may be a good outpatient candidate provided he is well, not dehydrated, and follow-up is assured.
The child with high-intermediate to high-risk for developing severe hyperbilirubinemia should be admitted for hydration, bililights, and/or assessment for exchange transfusion.
The unwell child with or without current neurologic findings should have immediate exchange transfusion.
References
Benitz WE. Hospital Stay for Healthy Term Newborn Infants. Pediatrics. 2015; 135(5):948-53.
Lauer BJ, Spector ND. Hyperbilirubinemia in the Newborn. Pediatrics in Review. 2011; 32(8):341-9.
This post and podcast are dedicated to Gita Pensa, MD, for her commitment to #FOAMed and passion for asynchronous learning and education innovation.
Children the world over are fascinated with what can possibly “fit” in their orifices. Diagnosis is often delayed. Anxiety abounds before and during evaluation and management.
Most common objects:1,2
| Food | Coins | Toys |
| Insects | Balls, marbles | Balloons |
| Magnets | Crayon | Hair accessories, bows |
| Beads | Pebbles | Erasers |
| Pen/marker caps | Button batteries | Plastic bags, packaging |
Non-pharmacologic techniques
Set the scene and control the environment. Limit the number of people in the room, the noise level, and minimize “cross-talk”. The focus should be on engaging, calming, and distracting the child.
Quiet room; calm parent; “burrito wrap”; guided imagery; have a willing parent restrain the child in his or her lap – an assistant can further restrain the head.
Procedural Sedation
Most foreign bodies in the ear, nose, and throat in children can be managed with non-pharmacologic techniques, topical aids, gentle patient protective restraint, and a quick hand. Consider sedation in children with special health care needs who may not be able to cooperate and technically delicate extractions. Ketamine is an excellent agent, as airway reflexes are maintained.3 Remember to plan, think ahead: where could the foreign body may be displaced if something goes wrong? You may have taken away his protective gag reflex with sedation. Position the child accordingly to prevent precipitous foreign body aspiration or occlusion.
L’OREILLE – DAS OHR – вухо – THE EAR – LA OREJA – 耳 – L'ORECCHIO
Essential anatomy: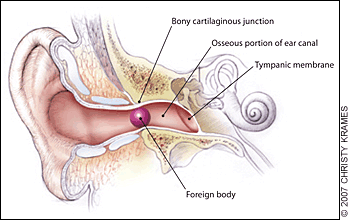
The external auditory canal. Foreign bodies may become lodged in the narrowing at the bony cartilaginous junction.4 The lateral 1/3 of the canal is flexible, while the medial 2/3 is fixed in the temporal bone – here is where many foreign bodies are lodged and/or where the clinician may find evidence of trauma.
Pearls:
- Ask yourself: is it graspable or non-graspable?5
- Graspable: 64% success rate, 14% complication rate
- Non-graspable: 45% success rate, 70% complication rate5
- If there is an insect in the external auditory canal, kill it first. They will fight for their lives if you try to dismember or take them out. “In the heat of battle, the patient can become terrorized by the noise and pain and the instrument that you are using is likely to damage the ear canal.”5,6 Use lidocaine jelly (preferred), viscous lidocaine (2%), lidocaine solution (2 or 4%), isopropyl alcohol, or mineral oil.
- Vegetable matter? Don’t irrigate it – the organic material will swell against the fixed structure, and cause more pain, make it much harder to extract, and may increase the risk of infection.
Pifalls:
- Failure to inspect after removal – is there something else in there?
- Failure to assess for abrasions, trauma, infection – if any break in skin, give prophylactic antibiotic ear drops
- Law of diminishing returns: probability of successful removal of ear foreign bodies declines dramatically after the first attempt
LE NEZ – DIE NASE – ніс – THE NOSE – LA NARIZ – 鼻 – IL NASO
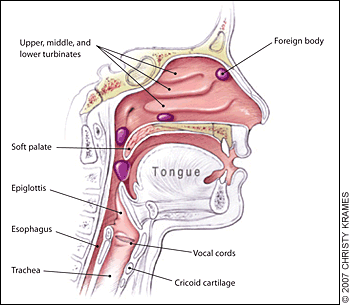 Essential anatomy:
Essential anatomy:
Nasopharyngeal and tracheal anatomy. Highlighted areas indicate points at which nasal foreign bodies may become lodged.4
Pearls:
- Consider using topical analgesics and vasoconstrictors to reduce pain and swelling – and improve tolerance of/cooperation with the procedure. Use 0.5% oxymetolazone (Afrin) spray and a few drops of 2 or 4% Pros: as above. Cons: possible posterior displacement of the foreign body.7
- Be ready for the precipitous development of an airway foreign body
Pitfalls:
- Beware of unilateral nasal discharge in a child – strongly consider retained foreign body.8
- Do not push a foreign body down the back of a patient's throat, where it may be aspirated into the trachea.
- Be sure to inspect the palate for “vacuum effect”: small or flexible objects may be found on the roof of the mouth, just waiting to be aspirated.
LA GORGE – DER HALS – горло – THE THROAT – LA GARGANTA – 喉 – LA GOLA
Before we go further –
Remember that a foreign body in the mouth or throat can precipitously become a foreign body in the airway. Foreign body inhalation is the most common cause of accidental death in children less than one year of age.9,10
Go to BLS maneuvers if the child decompensates.
Infants under 1 year of age – back blows: head-down, 5 back-blows (between scapulae), 5 chest-thrusts (sternum). Reassess, repeat as needed.
Children 1 year and up, conscious – Heimlich maneuver: stand behind patient with arms positioned under the patient’s axilla and encircling the chest. The thumb side of one fist should be placed on the abdomen below the xiphoid process. The other hand should be placed over the fist, and 5 upward-inward thrusts should be performed. This maneuver should be repeated if the airway remains obstructed. Alternatively, if patient is supine, open the airway, and if the object is readily visible, remove it. Abdominal thrusts: place the heel of one hand below the xiphoid, interlace fingers, and use sharp, forceful thrusts to dislodge. Be ready to perform CPR.
Children 1 year and up, unconscious – CPR: start CPR with chest compressions (do not perform a pulse check). After 30 chest compressions, open the airway. If you see a foreign body, remove it but do not perform blind finger sweeps because they may push obstructing objects further into the pharynx and may damage the oropharynx. Attempt to give 2 breaths and continue with cycles of chest compressions and ventilations until the object is expelled.
Chest films are limited: 80% of airway foreign bodies are radiolucent.11 Look for unilateral hyperinflation on expiratory films: air trapping.
Essential anatomy:
Most esophageal foreign bodies in children occur at the level of the thoracic inlet / cricopharyngeus muscle (upper esophageal sphincter). Other anatomically narrow sites include the level of the aortic arch and the lower esophageal sphincter.
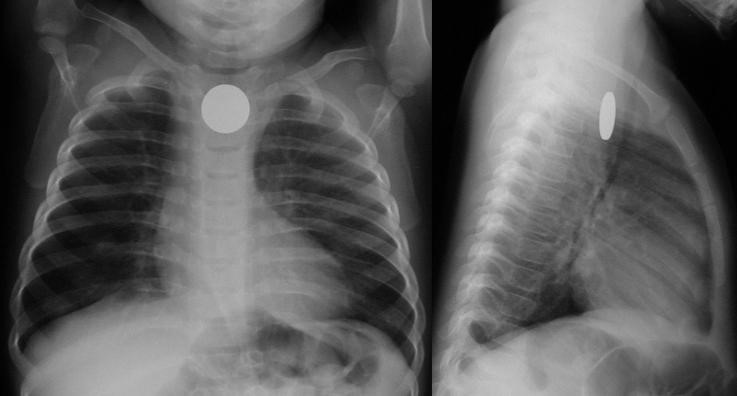
Coin en face – in the esophagus – lodged at the thoracic inlet.12 The pliable esophagus accommodates the flat coin against the flat aspect of the vertebra.11
Beware the “double-ring” sign: this is a button battery13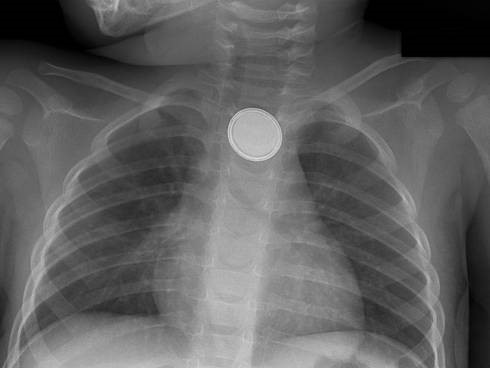
This is an emergency: the electrolyte-rich mucosa conducts a focal current from the narrow negative terminal of the battery, rapidly causing burn, necrosis, and possibly perforation. Emergent removal is required.
Button batteries that have passed into the stomach do not require emergent intervention – they can be followed closely.
Not a button battery, not a sharp object, not a long object?
If there is no obstruction, consider revaluation the next day – may wait up to 24 hours for passage.14 Sharieff et al.15 found that coins found in the mid to distal esophagus within 24 hours all passed successfully.
What conditions prompt urgent removal?
Size
Infants: objects smaller than 2 cm wide and 3 cm long will likely pass the pylorus and ileocecal valve10
Children and adults: objects smaller than 2 cm wide and 5 cm long will likely pass the pylorus and ileocecal valve9
Character
Sharp objects have a high rate of perforation (35%)1
Pearls:
- History is essential. Believe the parents and assume there is an aspirated/ingested foreign body until proven otherwise.
- History of choking, has persistent symptoms and/or abnormal xray? Broncoscopy! Cohen et al.16 found that of 142 patients evaluated at a single site university hospital, 61 had a foreign body. Of the 61 patients, 42 had abnormal physical exams and radiographs and 17 had either abnormal physical exams or radiographs, and 2 had normal physical exams and radiographs, but both had a history of persistent cough. Bottom line: history of choking PLUS abnormal exam, abnormal films, or persistent symptoms, evaluate with bronchoscopy.
- For patients with some residual suspicion of an aspirated foreign body (mild initial or improving symptoms; possibly abnormal chest x-ray; low but finite risk), consider chest CT with virtual bronchoscopy as a rule-out strategy.17,18
- Outpatients who have passed a small and non-concerning object into the stomach or beyond: serial exams and observing stools – polyethylene glycol 3350 (MiraLAX) may be given for delayed passage19
Pifalls:
- A single household magnet will likely pass through the GI tract, with the aforementioned dimensional caveats. Two or more magnets, however, run the risk of attraction and trans-bowel wall pressure necrosis.
- Not all magnets are created equal. Neodymium magnet toys (“buckyballs”) were recalled in 2014 (but are still out there!) due to their highly attractive nature. These magnets must be removed to avoid bowel wall ischemia. 19–21
- Patients should avoid wearing belt buckles or metallic buttons in case of single magnet ingestion while waiting for the single magnet to pass

DES OUTILS DU MÉTIER – DIE HANDWERKSZEUG – Знаряддя праці
– TOOLS OF THE TRADE –
LAS HERRAMIENTAS DEL OFICIO – GLI ATTREZZI DEL MESTIERE – 仕事のツール
It’s best to keep your armamentarium as large as you can.
Curette
A small foreign body in the lateral 1/3 of the auditory canal may be amenable to a simple curettage. Hair beads (if the central hole is accessible) may be manipulated out with the angled tip of a rigid curette. Steady the operating hand by placing your hypothenar eminence on the child’s zygoma or temporal scalp, to avoid jutting the instrument into the ear canal with sudden movement. There is a large selection of disposable simple and lighted curettes on the market.
Right-angle Hook
Various eponymous hooks are available for this purpose; one in popular use is the Day hook, which may be passed behind the foreign body.22 An inexpensive and convenient alternative to the commercially available right-hooks is a home-made version: make your own by straightening out a paperclip and bending it to a right angle23 at 2-3 mm from the end (be sure not to use the type that have a friable shiny metallic finish, as the residue may be left behind in the ear canal). If it is completely lodged, use of a right-angle hook will likely only cause trauma to the canal.
Alligator forceps
Alligator forceps are best for grasping soft objects like cotton or paper. Smooth, round or oval objects are not amenable to extraction with alligator forceps. When using them, be sure to get a firm, central grip on the object, to avoid tearing it into smaller, less manageable pieces.
Pro tip: Look before you grip! Be careful to visualize the area you are gripping, to avoid pulling on (and subsequently avulsing) soft tissue in the ear canal.
Cyanoacrylate (Dermabond®, SurgiSeal®)
Apply cyanoacrylate to either side of a long wooden cotton swab (the lecturer prefers the cotton tip side, for improved grip/molding around object). Immediately apply the treated side to the object in the ear canal in a restrained patient. Steady the hypothenar eminence on the child’s face to avoid dislodgement of the cotton swab with sudden movement. Apply the treated swab to the foreign body for 30-60 seconds, to allow bonding. Slowly pull out the foreign body. Re-visualize the ear canal for other retained foreign bodies and abrasion or ear canal trauma.
Did the cyanoacrylate drip? Did the swab stick to the ear canal?
No problem – use 3% hydrogen peroxide or acetone.24 Pour in a sufficient amount, allow to work for 10 minutes. Both agents help to dissolve ear wax, the compound, or both. Repeat if needed to debond the cyanoacrylate from the ear canal.24,25
Irrigation
Irrigation is the default for non-organic foreign bodies that are not amenable to other extraction techniques. Sometimes the object is encased in cerumen, and the only “instrument” that will fit behind the foreign body is the slowly growing trickle of water that builds enough pressure to expulse it. Do not use if there is any organic material involved: the object will swell, causing much more pain, difficulty in extraction, and possibly setting up conditions for infection.
Position the child either prone or supine, gently restrain (as above). Let gravity work for you: don’t irrigate in decubitus position with the affected ear up. It may be more accessible to you, but you may never get the foreign body out.
To use a butterfly needle: use a small gage (22 or 24 g) butterfly set up, cut off the needle, connect the tubing to a 30 mL syringe filled with warm or room-temperature water. Insert the free end of the tubing gently, and “secure” the tubing with your pinched fingers while irrigating (think of holding an ETT in place just after intubation and before taping/securing the tube). Gently and slowly increase the pressure you exert as you irrigate.
To use an IV or angiocatheter: use a moderate size (18 or 20 g) IV, remove the needle and attach the plastic catheter to a 20 mL syringe, and irrigate as above.
Acetone
Acetone has been used successfully to remove chewing gum, Styrofoam, and superglue from the ear canal24,26,27 Use in cases where there is no suspicion of perforation of the tympanic membrane.
Docusate Sodium (Colace®)
Cerumen is composed of sebaceous ad ceruminous secretions and desquamated skin. Genetic, environmental, and anatomical factors combine to trap a foreign body in the external canal. Use of a ceruminolytic such as docusate sodium may help to loosen and liberate the foreign body.28 Caveat medicus: Adding docusate sodium will make the object more slippery – this may or may not be an issue given the nature of the foreign body.
In the case where loosening the ear wax may aid extraction (and will not cause a slippery mess), consider filling the ear canal will docusate sodium (Colace), having the child lie with the affected side up, waiting 15 minutes, and proceeding. This is especially helpful when planning for irrigation.
Magnets
Rare earth magnets (a misnomer, as their components are actually abundant) such as neodymium can be useful in retrieving metallic foreign bodies (e.g. button batteries in the nose or ears).29,30 Magnetic “pick-up tools” – used by mechanics, engineers, and do-it-yourselfers – are inexpensive and readily available in various sizes, shapes, and styles such as a telescoping extender. Look for a small tip diameter (to fit in the ear canal as well as the nose) and a strong “hold” (at least a 3-lb hold).
Alternatively, you may purchase a strong neodymium bar magnet (30- to 50-lb hold) to attach to an instrument such as an alligator forceps, pick-up forceps, or small hemostat (a pacemaker magnet may also work). The magnetic bar, placed in your palm at the base of the instrument, will conduct the charge (depending on the instrument) and allow you to retrieve many metallic objects.31 Although stainless steel is often said to be “non-magnetic”, it depends on the alloy used, and some may actually respond to the strong rare earth magnet. Most stainless steel has a minimum of 10.5% chromium, which gives the steel its 'stainless' properties (essentially corrosion resistance). A basic stainless steel has a “ferritic” structure and is magnetic. Higher-end stainless steel such as in kitchen cutlery have an “austenitic” structure, with more chromium added, and so less magnetic quality. (Ironically, the more “economical” instruments in the typical ED suture kit have less chromium, and so are more magnetic – use these with your neodymium bar magnet to conduct the magnetic charge and extract the metallic foreign body.)
Bottom line: if it’s metal, it’s worth a try to use a magnet. Even if the metal is very weakly magnetic, the strong neodymium magnet may still be able to exert a pull on it and aid in extraction.
Snare Technique
A relatively new method, described by Fundakowski et al.32 consists of using a small length of 24-gauge (or similar) wire (available inexpensively online, and kept in your personal “toolkit”; see Appendix B below) to make a loop that is secured by a hemostat (the 24-gauge wire is easily cut into strips with standard trauma scissors). After treatment with oxymetolazone (0.05%) and lidocaine (1 or 2%) topically, the loop is passed behind the foreign body (in the case report, a button battery). Pull the loop toward you until you feel that it is sitting up against the button battery. Now, turn the hemostat 90° to improve your purchase on the foreign body. Pull gently out. This technique is especially useful when the foreign body has created marked edema, either creating a vacuum effect or making it difficult for other instruments to pass.
Balloon Catheters (Katz extractor®, Fogarty embolectomy catheter)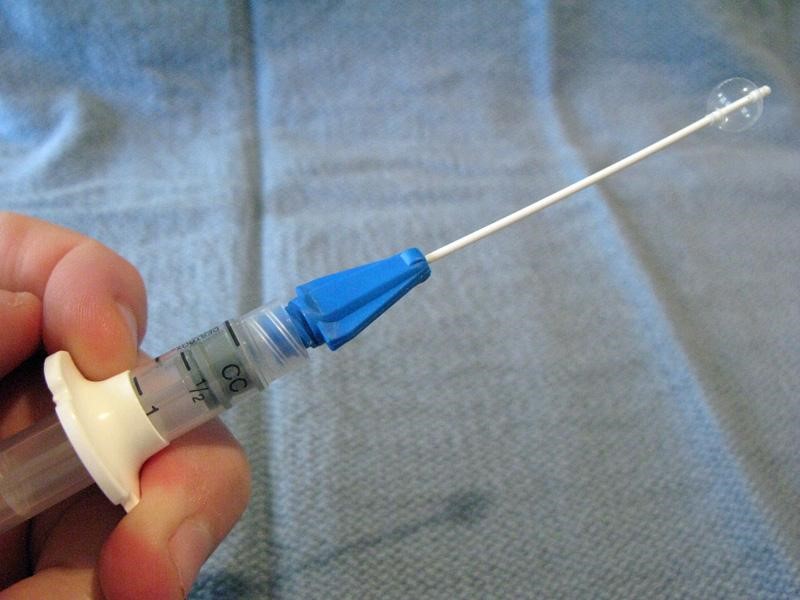
Small-caliber devices (5, 6, or 8 F) originally designed for use with intravascular or bladder catheterization may be used to extract foreign bodies from the ear or nose.7,33 A catheter designed specifically for foreign body use is the Katz extractor. Inspect the ear or nose for potential trauma and to anticipate bleeding after manipulation (especially the nose). Deflate the catheter and apply surgical lubricant or 2% lidocaine jelly. Insert the deflated catheter and gently pass the device past the foreign body. Inflate the balloon and slowly pull the balloon and foreign body out. Re-inspect after extraction.
NB, from the manufacturer of the Katz extractor, InHealth: “the Katz Extractor oto-rhino foreign body remover is intended principally for extraction of impacted foreign bodies in the nasal passages. This device may also be used in the external ear canal, based upon clinical judgment.”
Mother’s kiss
 The mother’s kiss was first described in 1965 by Vladimir Ctibor, a general practitioner from New Jersey.34 “The mother, or other trusted adult, places her mouth over the child’s open mouth, forming a firm seal as if about to perform mouth-to-mouth resuscitation. While occluding the unaffected nostril with a finger, the adult then blows until feeling resistance caused by closure of the child’s glottis, at which point the adult gives a sharp exhalation to deliver a short puff of air into the child’s mouth. This puff of air passes through the nasopharynx, out through the non-occluded nostril and, if successful, results in the expulsion of the foreign body. The procedure is fully explained to the adult before starting, and the child is told that the parent will give him or her a “big kiss” so that minimal distress is caused to the child. The procedure can be repeated a number of times if not initially successful.”34
The mother’s kiss was first described in 1965 by Vladimir Ctibor, a general practitioner from New Jersey.34 “The mother, or other trusted adult, places her mouth over the child’s open mouth, forming a firm seal as if about to perform mouth-to-mouth resuscitation. While occluding the unaffected nostril with a finger, the adult then blows until feeling resistance caused by closure of the child’s glottis, at which point the adult gives a sharp exhalation to deliver a short puff of air into the child’s mouth. This puff of air passes through the nasopharynx, out through the non-occluded nostril and, if successful, results in the expulsion of the foreign body. The procedure is fully explained to the adult before starting, and the child is told that the parent will give him or her a “big kiss” so that minimal distress is caused to the child. The procedure can be repeated a number of times if not initially successful.”34
Positive Pressure Ventilation with Bag Valve Mask
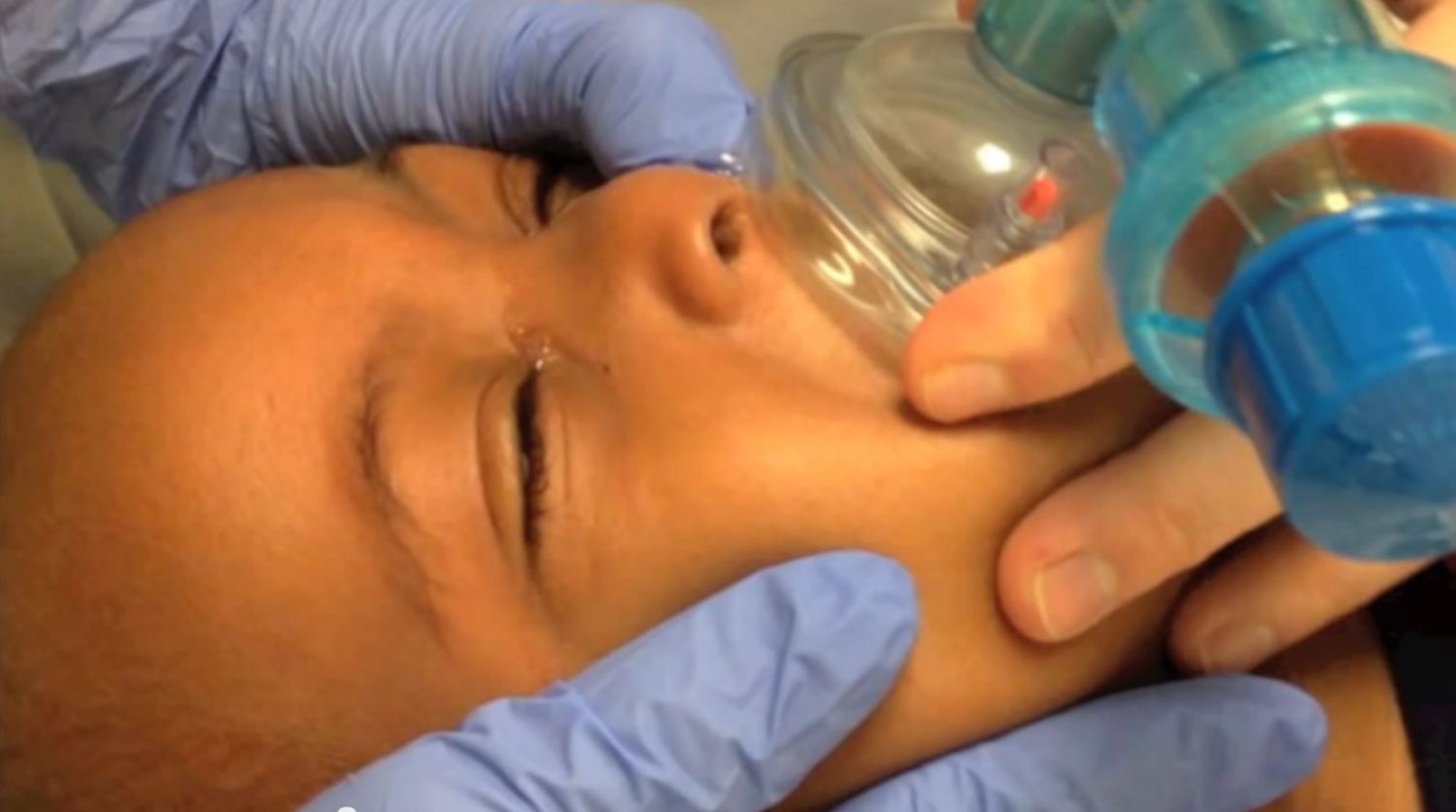 This technique is an approximation of the above mother’s kiss technique – useful for unwilling parents or unsuccessful tries.10,25 The author prefers to position the child sitting up. A self-inflating bag-mask device is fitted with a very small mask: use an abnormally small mask (otherwise inappropriately small for usual resuscitative bag-mask ventilation) to fit over the mouth only. Choose an infant mask that will cover the child’s mouth only. Occlude the opposite nostril with your finger while you form a tight seal with the mask around the mouth. Deliver short, abrupt bursts of ventilation through the mouth – be sure to maintain good seals with the mask and with your finger to the child’s nostril – until the foreign body is expulsed through the affected nostril.
This technique is an approximation of the above mother’s kiss technique – useful for unwilling parents or unsuccessful tries.10,25 The author prefers to position the child sitting up. A self-inflating bag-mask device is fitted with a very small mask: use an abnormally small mask (otherwise inappropriately small for usual resuscitative bag-mask ventilation) to fit over the mouth only. Choose an infant mask that will cover the child’s mouth only. Occlude the opposite nostril with your finger while you form a tight seal with the mask around the mouth. Deliver short, abrupt bursts of ventilation through the mouth – be sure to maintain good seals with the mask and with your finger to the child’s nostril – until the foreign body is expulsed through the affected nostril.
Beamsley Blaster (Continuous Positive Pressure) Technique
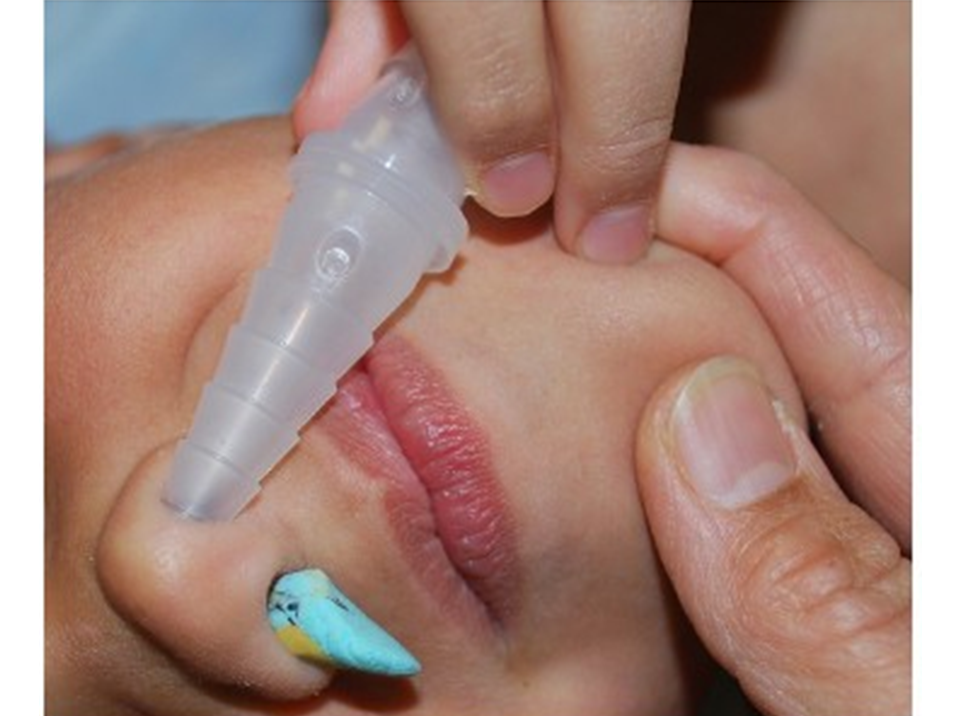 For the very uncooperative child with a nasal foreign body amenable to positive pressure ventilation who fails the mother’s kiss and bag-mask technique, a continuous positive pressure method may be used. Connect one end of suction tubing to the male adaptor (“Christmas tree”) of an air or oxygen source. Connect the other end of the suction tubing to a male-to-male adaptor (commonly used for chest tube connections or connecting / extending suction tubes). Insert the end of the device into the child’s unaffected nostril. The air flow will deliver positive pressure ventilation continuously.
For the very uncooperative child with a nasal foreign body amenable to positive pressure ventilation who fails the mother’s kiss and bag-mask technique, a continuous positive pressure method may be used. Connect one end of suction tubing to the male adaptor (“Christmas tree”) of an air or oxygen source. Connect the other end of the suction tubing to a male-to-male adaptor (commonly used for chest tube connections or connecting / extending suction tubes). Insert the end of the device into the child’s unaffected nostril. The air flow will deliver positive pressure ventilation continuously.
With this technique there is a theoretical risk of barotrauma to the lungs or tympanic membranes. However, there is only one case reported in the literature of periorbital subcutaneous emphysema.
To minimize this risk, some authors recommend limiting to a maximum of four attempts using any positive pressure method.10
Nasal speculum
Optimize your visualization with a nasal speculum. The nostrils, luckily, will accommodate a fair amount of distention without damage.
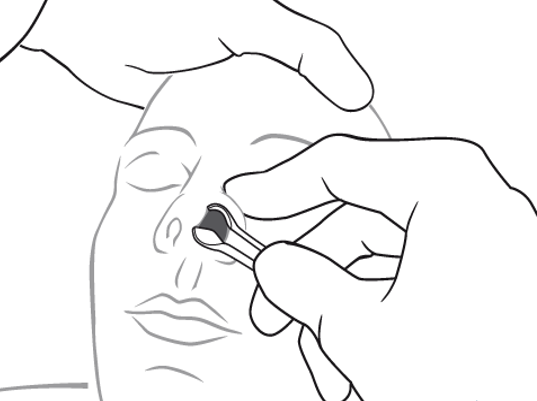 Hold the speculum vertically to avoid pressure on and damage to the vessel-and-nerve-rich nasal septum. Hold the handle of the speculum in the palm of your hand comfortably and while placing your index finger on the patient’s ala. This will help to control the speculum and your angle of sight. Your other hand is then free to use a hook or other tool for extraction.
Hold the speculum vertically to avoid pressure on and damage to the vessel-and-nerve-rich nasal septum. Hold the handle of the speculum in the palm of your hand comfortably and while placing your index finger on the patient’s ala. This will help to control the speculum and your angle of sight. Your other hand is then free to use a hook or other tool for extraction.
Lighting is especially important when using the nasal speculum: a focused procedure light or head lamp is very helpful. The author keeps a common camping LED headlamp in his bag for easy access.
Suction tips / catheters
Various commercial and non-commercial suction devices are on the market for removal of foreign bodies. All connect to wall suction, and vary by style, caliber of suction, and tip end interface. A commonly available suction catheter is the Frazier suction tip (right), a single-use device used in the operating room.
A modification to suction can be made with the Schuknecht foreign body remover (left; not to be confused with the suction catheter of the same name): a plastic cone-shaped tip placed on the end of the suction catheter to increase vacuum surface area and seal.
Laryngoscope and Magill Forceps
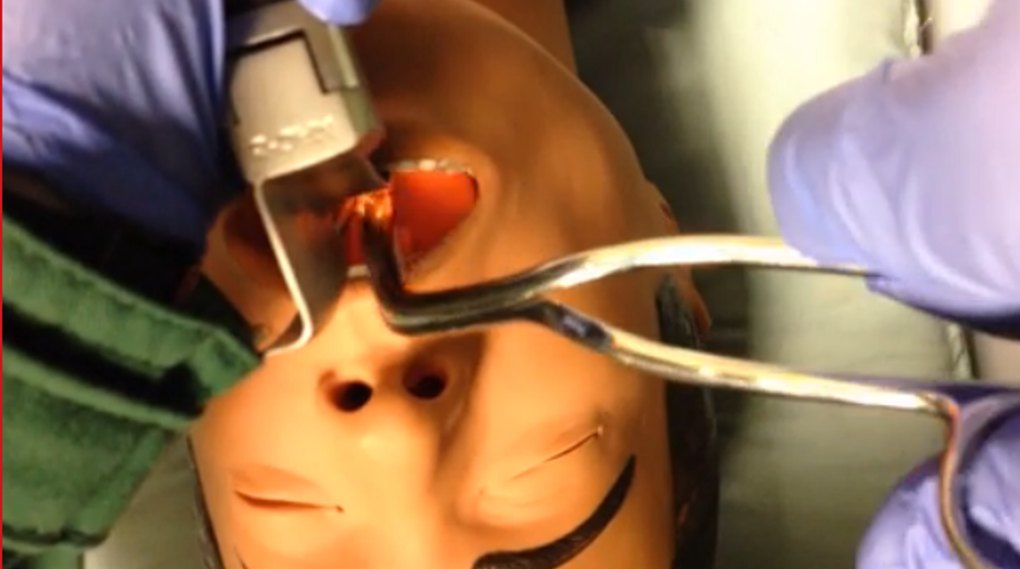 If a child aspirates and occludes his airway, return to BLS maneuvers (as above). If the child becomes obtunded, use direct laryngoscopy to visualize the foreign body and remove with the Magill forceps. Hold the laryngoscope in your left hand as per usual. Hold the Magill forceps in your right hand – palm side down – to grasp and remove the foreign body.
If a child aspirates and occludes his airway, return to BLS maneuvers (as above). If the child becomes obtunded, use direct laryngoscopy to visualize the foreign body and remove with the Magill forceps. Hold the laryngoscope in your left hand as per usual. Hold the Magill forceps in your right hand – palm side down – to grasp and remove the foreign body.
Take-home Points
Beware the “vacuum palate”: a flat (especially clear plastic) foreign body hiding on the palate
Take seriously the complaint of foreign body without obvious evidence on initial inspection – believe that something is in there until proven otherwise
Control the environment, address analgesia and anxiolysis, have a back-up plan
Motto
Like a difficult airway: plan through the steps
MERCI – DANKE – Дякую – THANK YOU – GRACIAS – ありがとう— GRAZIE
Appendix A: Prevention
At the end of the visit, after some rapport has been established, counsel the caregivers about age-appropriate foods and “child-proofing” the home. This is a teachable moment – and only you can have this golden opportunity!
Age-appropriate foods
0-6 months: breastmilk or formula
6-9 months: introduce solid puree-consistency foods
9-12 months: small minced solids that require no chewing (well cooked, soft, chopped foods)
Although molars (required for chewing) erupt around 18 months, toddlers need to develop coordination, awareness to eat hard foods that require considerable chewing.
Not until 4 years of age (anything that requires chewing to swallow):
Hot dogs
Nuts and seeds
Chunks of meat or cheese
Whole grapes
Hard or sticky candy
Popcorn
Chunks of peanut butter
Chunks of raw vegetables
Chewing gum
Child-proofing the home
Refer parents to the helpful multi-lingual site from the American Academy of Pediatrics:
http://www.healthychildren.org/English/safety-prevention/at-home/Pages/Childproofing-Your-Home.aspx
An abbreviated list: use age-appropriate toys and “test” them out before giving them to your child to verify that there are no small, missing, or loose parts. Secure medications, lock up cabinets (especially with chemicals), do not store chemicals in food containers, secure the toilet bowl, and unplug appliances.
Parents should understand that “watching” their child alone cannot prevent foreign body aspiration: a recent review found that in 84.2% of cases, incidents resulting in an airway foreign body occurred in the presence of an adult.35
Best overall tip: get down on all fours and inspect your living area from the child’s perspective. It is amazing what you will find when you are least expecting it.
Appendix B: The Playbook's ENT Foreign Body Toolkit
Although your institution should supply you with what you need to deal with routine problems, we all struggle with having just what we need when we need it. High-volume disposable items such as cyanoacrylate (Dermabond), curettes, supplies for irrigation, alligator forceps, and the like certainly should be supplied by the institution. However, some things come in very handy as our back-up tools.
NB: we should be cognizant of the fact that tools that must be sterilized or autoclaved are not good candidates for our personal re-usable toolkits.
These items can all be found inexpensively – shop around online, or in home improvement stores:
- Head lamp, LED camping style: $5-15
- Neodymium magnet “pick-up tool”: $5-15
- Neodymium bar magnet: $6-20
- Wire, 24-gauge, spool of 25 yards (for snare technique): $6
- Day hook: $15-20
References
- Chapin MM, Rochette LM, Annest JL, Haileyesus T, Conner KA, Smith GA. Nonfatal Choking on Food Among Children 14 Years or Younger in the United States, 2001–2009. Pediatrics. 2013;132(2):275-281. doi:10.1542/peds.2013-0260.
- Committee on Injury V. Policy Statement—Prevention of Choking Among Children. Pediatrics. 2010:peds.2009-2862. doi:10.1542/peds.2009-2862.
- Brown L, Denmark TK, Wittlake WA, Vargas EJ, Watson T, Crabb JW. Procedural sedation use in the ED: management of pediatric ear and nose foreign bodies. Am J Emerg Med. 2004;22(4):310-314.
- Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76(8):1185-1189.
- DiMuzio J, Deschler DG. Emergency department management of foreign bodies of the external ear canal in children. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2002;23(4):473-475.
- Leffler S, Cheney P, Tandberg D. Chemical immobilization and killing of intra-aural roaches: an in vitro comparative study. Ann Emerg Med. 1993;22(12):1795-1798.
- Kiger JR, Brenkert TE, Losek JD. Nasal foreign body removal in children. Pediatr Emerg Care. 2008;24(11):785-792; quiz 790-792. doi:10.1097/PEC.0b013e31818c2cb9.
- Kadish HA, Corneli HM. Removal of nasal foreign bodies in the pediatric population. Am J Emerg Med. 1997;15(1):54-56.
- Tahir N, Ramsden WH, Stringer MD. Tracheobronchial anatomy and the distribution of inhaled foreign bodies in children. Eur J Pediatr. 2009;168(3):289-295. doi:10.1007/s00431-008-0751-9.
- Rempe B, Iskyan K, Aloi M. An Evidence-Based Review of Pediatric Retained Foreign Bodies. Pediatr Emerg Med Pract. 6(12).
- Digoy GP. Diagnosis and management of upper aerodigestive tract foreign bodies. Otolaryngol Clin North Am. 2008;41(3):485-496, vii - viii. doi:10.1016/j.otc.2008.01.013.
- Loren Yamamoto, Inaba A, DiMauro R. Radiologic Cases in Pediatric Emergency Medicine; University of Hawaii. Radiol Cases Emerg Med. http://www.hawaii.edu/medicine/pediatrics/pemxray/zindex.html. Accessed February 20, 2015.
- Painter K. Energizer makes button battery packages safer for kids. USA Today.
- ASGE Standards of Practice Committee, Ikenberry SO, Jue TL, et al. Management of ingested foreign bodies and food impactions. Gastrointest Endosc. 2011;73(6):1085-1091. doi:10.1016/j.gie.2010.11.010.
- Sharieff GQ, Brousseau TJ, Bradshaw JA, Shad JA. Acute esophageal coin ingestions: is immediate removal necessary? Pediatr Radiol. 2003;33(12):859-863. doi:10.1007/s00247-003-1032-4.
- Cohen S, Avital A, Godfrey S, Gross M, Kerem E, Springer C. Suspected Foreign Body Inhalation in Children: What Are the Indications for Bronchoscopy? J Pediatr. 2009;155(2):276-280. doi:10.1016/j.jpeds.2009.02.040.
- Haliloglu M, Ciftci AO, Oto A, et al. CT virtual bronchoscopy in the evaluation of children with suspected foreign body aspiration. Eur J Radiol. 2003;48(2):188-192. doi:10.1016/S0720-048X(02)00295-4.
- Jung SY, Pae SY, Chung SM, Kim HS. Three-dimensional CT with virtual bronchoscopy: a useful modality for bronchial foreign bodies in pediatric patients. Eur Arch Otorhinolaryngol. 2011;269(1):223-228. doi:10.1007/s00405-011-1567-1.
- Hussain SZ, Bousvaros A, Gilger M, et al. Management of ingested magnets in children. J Pediatr Gastroenterol Nutr. 2012;55(3):239-242. doi:10.1097/MPG.0b013e3182687be0.
- Brown JC, Otjen JP, Drugas GT. Too attractive: the growing problem of magnet ingestions in children. Pediatr Emerg Care. 2013;29(11):1170-1174. doi:10.1097/PEC.0b013e3182a9e7aa.
- Brown JC, Otjen JP, Drugas GT. Pediatric magnet ingestions: the dark side of the force. Am J Surg. 2014;207(5):754-759; discussion 759. doi:10.1016/j.amjsurg.2013.12.028.
- Menner AL. Pocket Guide to the Ear: A Concise Clinical Text on the Ear and Its Disorders. Thieme; 2011.
- Colina D, Dudek S, Lin M. Tricks of the Trade: ENT Dilemmas - How Do I Get That Out of There? ACEP News. http://www.acep.org/Clinical---Practice-Management/Tricks-of-the-Trade--ENT-Dilemmas---How-Do-I-Get-That-Out-of-There-/?__taxonomyid=118010. Published July 2009. Accessed February 5, 2015.
- Abadir WF, Nakhla V, Chong P. Removal of superglue from the external ear using acetone: case report and literature review. J Laryngol Otol. 1995;109(12):1219-1221.
- Kadish H. Ear and Nose Foreign Bodies “It is all about the tools.” Clin Pediatr (Phila). 2005;44(8):665-670. doi:10.1177/000992280504400803.
- Chisholm EJ, Barber-Craig H, Farrell R. Chewing gum removal from the ear using acetone. J Laryngol Otol. 2003;117(4):325. doi:10.1258/00222150360600995.
- White SJ, Broner S. The use of acetone to dissolve a Styrofoam impaction of the ear. Ann Emerg Med. 1994;23(3):580-582.
- Singer AJ, Sauris E, Viccellio AW. Ceruminolytic effects of docusate sodium: a randomized, controlled trial. Ann Emerg Med. 2000;36(3):228-232. doi:10.1067/mem.2000.109166.
- Bledsoe RD. Magnetically adherent nasal foreign bodies: a novel method of removal and case series. Am J Emerg Med. 2008;26(7):839.e1-e839.e2. doi:10.1016/j.ajem.2008.01.036.
- Dolderer JH, Kelly JL, Morrison WA, Penington AJ. FOREIGN-BODY RETRIEVAL USING A RARE EARTH MAGNET: Plast Reconstr Surg. 2004;113(6):1869-1870. doi:10.1097/01.PRS.0000119869.01081.1C.
- Yeh B, Roberson JR. Nasal magnetic foreign body: a sticky topic. J Emerg Med. 2012;43(2):319-321. doi:10.1016/j.jemermed.2010.02.013.
- Fundakowski CE, Moon S, Torres L. The snare technique: a novel atraumatic method for the removal of difficult nasal foreign bodies. J Emerg Med. 2013;44(1):104-106. doi:10.1016/j.jemermed.2012.07.070.
- Chan TC, Ufberg J, Harrigan RA, Vilke GM. Nasal foreign body removal. J Emerg Med. 2004;26(4):441-445. doi:10.1016/j.jemermed.2003.12.024.
- Cook S, Burton M, Glasziou P. Efficacy and safety of the “mother’s kiss” technique: a systematic review of case reports and case series. Can Med Assoc J. 2012;184(17):E904-E912. doi:10.1503/cmaj.111864.
- Gregori D, Morra B, Snidero S, et al. Foreign bodies in the upper airways: the experience of two Italian hospitals. J Prev Med Hyg. 2007;48(1):24-26.
This post and podcast are dedicated to Linda Dykes, MBBS(Hons) for her can-do attitude and collaborative spirit. Thank you for sharing your knowledge, experience, and heart with the world.
When you give only after you're asked, you've waited too long.
– John Mason
First, learn to bag
Place a towel roll under the scapulae to align oral, pharyngeal, and tracheal axes:
Use airway adjuncts such as the oropharyngeal airway or a nasal trumpet.
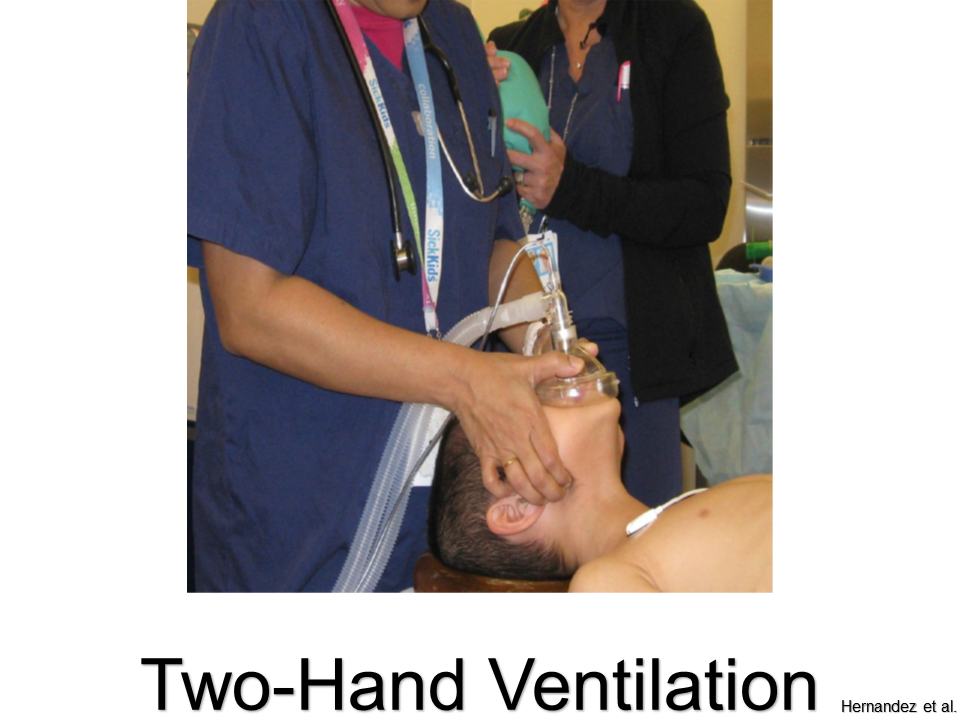
Use the two-hand ventilation technique whenever possible:
(See Adventures in RSI for more)
Supraglottic Airways:
for difficult bag-valve-mask ventilation or a difficult airway
(details in audio)
LMA Classic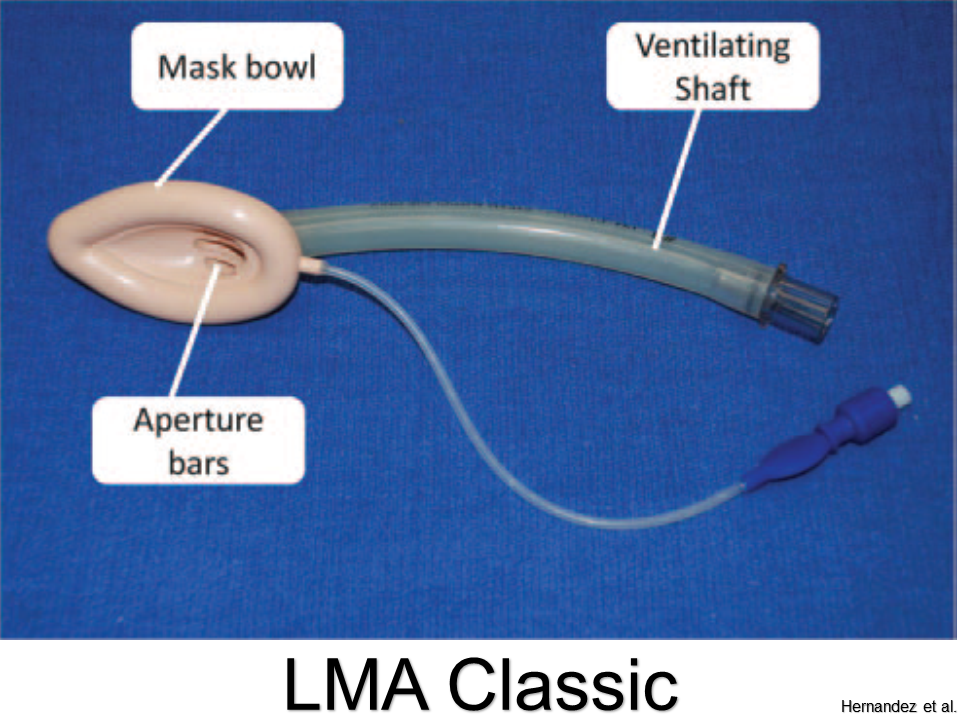
Pros: Best studied; sizes for all ages
Cons: Cannot intubate through aperture
LMA Supreme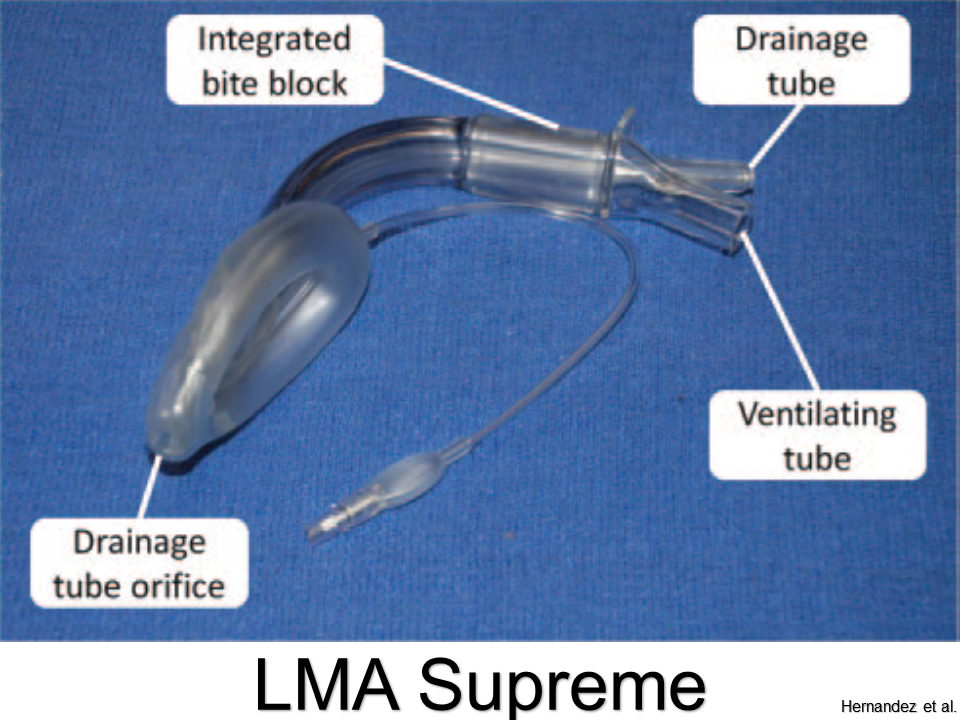
Pros: Better ergonomics with updated design; bite bloc; port for decompression
Cons: Cannot pass appropriate-sized ETT through tube
King Laryngeal Tube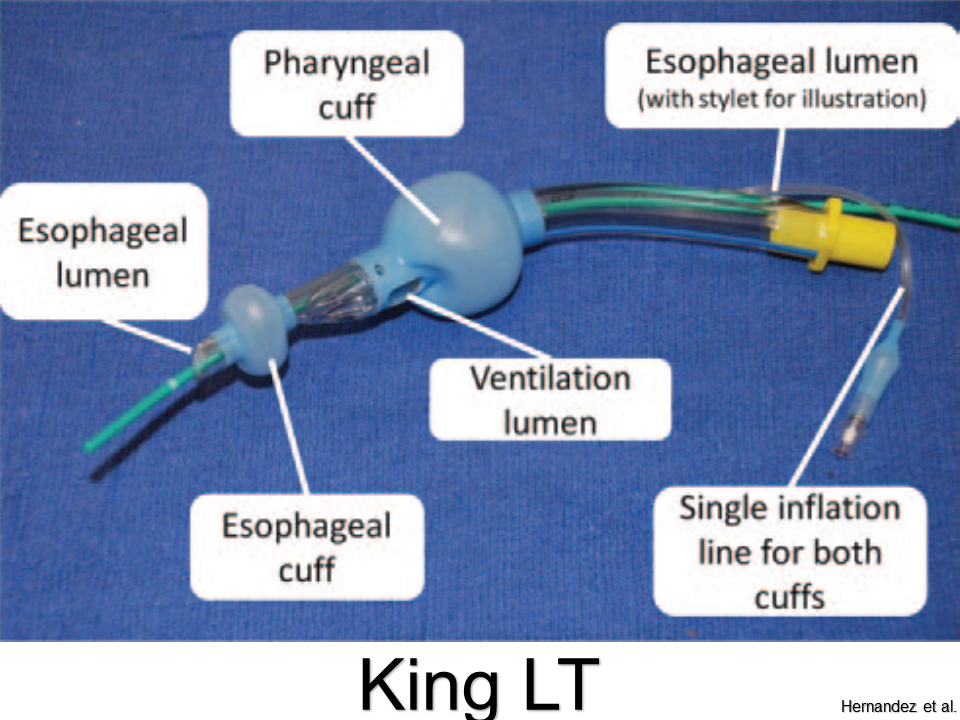
Pros: Little training needed; high success rate; single inflation port
Cons: Flexion of tube can impede ventilation or cause leaks; only sized down to 12 kg (not for infants and most toddlers)
Air-Q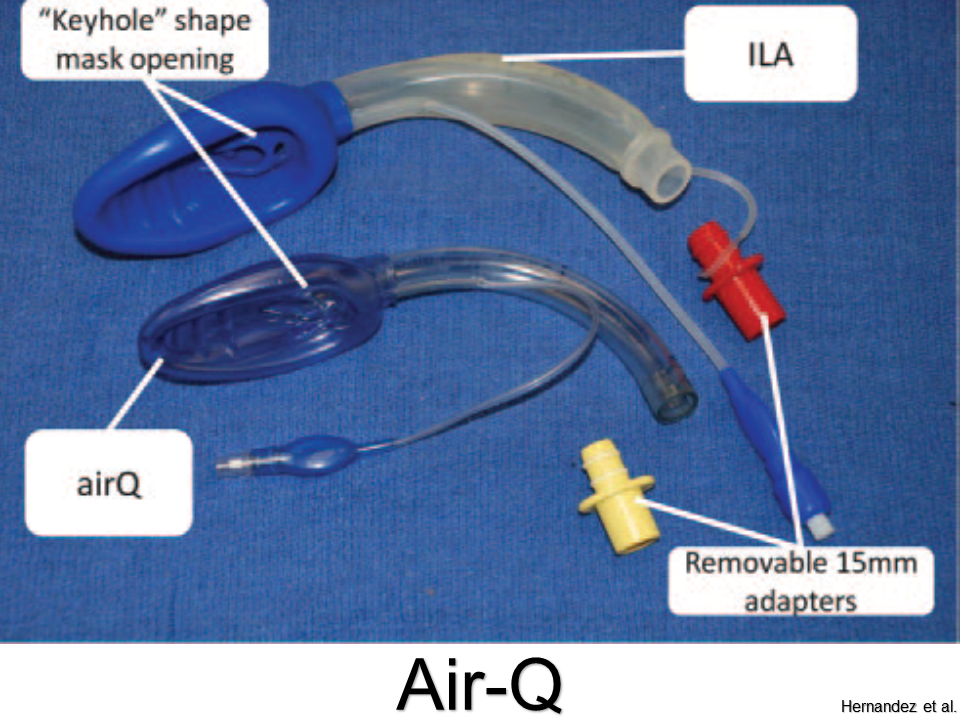
Pros: Easy to place; can intubate through aperture
Cons: Not for neonates less than 4 kg
iGel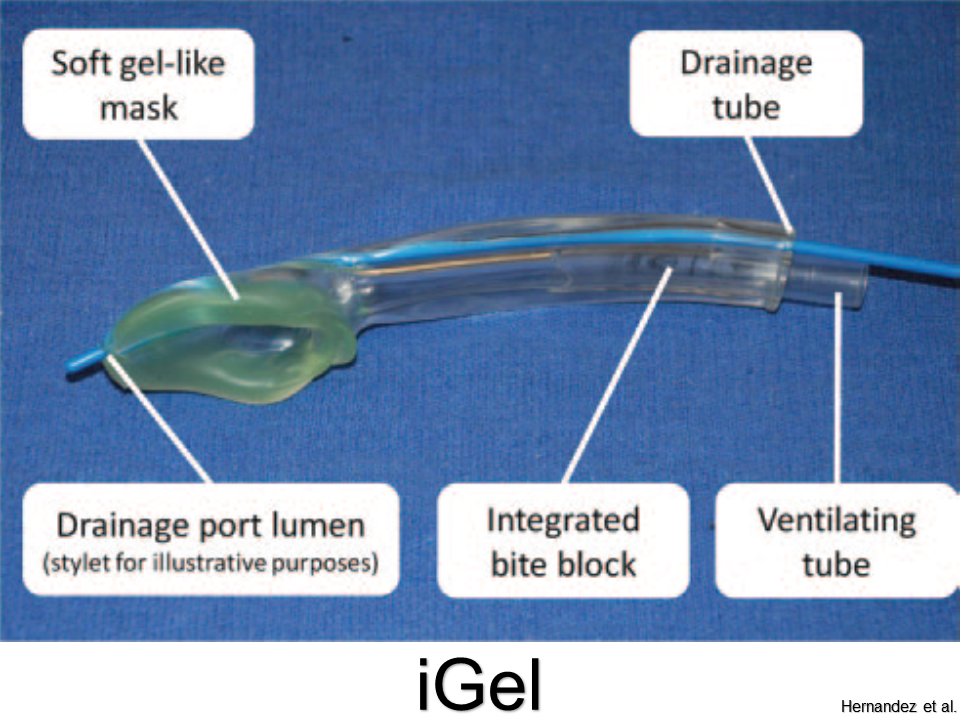
Pros: Molds more accurately to supraglottis; no need to inflate; good seal pressures
Cons: Cannot intubate through (without fiberoscopy)
Summary
• If you can bag the patient, you're winning.
• If you have difficulty bagging, or anticipate or encounter a difficult airway, then don't forget your friend the supraglottic airway (SGA).
• Ego is the enemy of safety: SGAs are simple, fast, and reliable.
• Just do it.
References
Supraglottic Airway on WikEM
This post and podcast are dedicated to Tim Leeuwenburg, MBBS FRACGP FACRRM DRANZCOG DipANAES and Rich Levitan, MD, FACEP for keeping our minds and our patients' airways -- open. You make us better doctors. Thank you.
Powered by #FOAMed — Tim Horeczko, MD, MSCR, FACEP, FAAP
Pediatric; Emergency Medicine; Pediatric Emergency Medicine; Podcast; Pediatric Podcast; Emergency Medicine Podcast; Horeczko; Harbor-UCLA; Presentation Skills; #FOAMed #FOAMped #MedEd
When should you commit to getting urine?
When can you wait?
When should you forgo testing altogether?
When do I get urine?
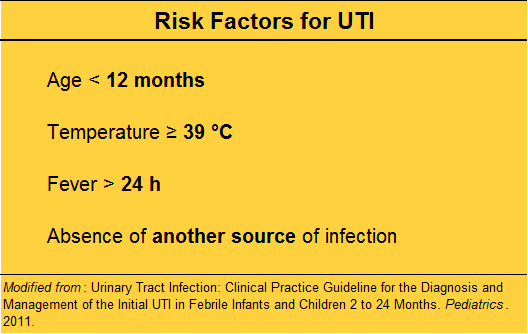
Symptoms – either typical dysuria, urgency, frequency in a verbal child, or non-descript abdominal pain or vomiting in a well appearing child.
Fever – but first look for an obvious alternative source, especially viral signs or symptoms.
No obvious source?
Risk stratify before “just getting a urine”.
In a low risk child, with obviously very vigilant parents, who is well appearing, you may choose not to test now, and ensure close follow up.
Bag or cath?
The short answer is: always cath, never bag.
(Pros and cons in audio)
What is the definition of a UTI?
According to the current clinical practice guideline by the AAP, the standard definition of a urinary tract infection is the presence of BOTH pyuria AND at least 50 000 colonies per mL of a single uropathogen.
Making the diagnosis in the ED:
The presence of WBCs with a threshold of 5 or greater WBCs per HPF is required.
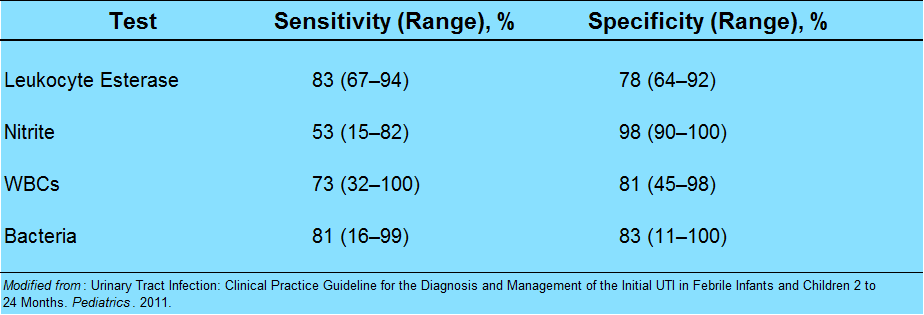
What else goes into the urinalysis that may be helpful?
Pearl: nitrites are poorly sensitive in children. It takes 4 hours for nitrites to form, and most children this age do no hold their urine.
Pearl: the enhanced urinalysis is the addition of a gram stain. A positive gram stain has a LR+ of 87 in infants less than 60 days, according to a study by Dayan et al. in Pediatric Emergency Care.
When can I just call it pyelonephritis?
In an adult, we look for UTI plus evidence of focal upper tract involvement, like CVA tenderness to percussion or systemic signs like nausea, vomiting, or fever. It is usually straightforward.
It’s for this reason that the literature uses the term “febrile UTI” for children. Fever is very sensitive, but not specific in children.
The ill-appearing child has pyelonephritis. The well-appearing child likely has a “febrile UTI”, without upper involvement. However, undetected upper tract involvement may be made in retrospect via imaging, if done.
How should I treat UTIs?
For simple lower tract disease, treat for at least 7 days. There is no evidence to support 7 versus 10 versus 14 days. My advice: use 7-10 days as your range for simple febrile UTI in children.
Pyelonephritis should be treated for a longer duration. Treat pyelonephritis for 10-14 days.
What should we give them?
Sulfamethoxazole and trimethoprim (Bactrim) is falling out of favor, mostly because isolates in many communities are resistant. There is an association of Stevens-Johnson Syndrome (SJS) with Bactrim use. This may be confounded by its prior popularity; any antibiotic can cause SJS, but there are more case reports with Bactrim.
Cephalexin (Keflex): 25 mg/kg dose, either BID or TID. It is easy on the stomach, rarely interacts with other meds, has high efficacy against E. coli, and most importantly, cephalexin has good parenchymal penetration.
Nitrofurantoin is often used in pregnant women, because the drug tends to concentrate locally in the urine. However, blood and tissue concentrations are weak. It may be ineffective if there is some sub-clinical upper tract involvement.
Cefdinir is a 3rd generation cephalosporin available by mouth, given at 14 mg/kg in either one dose daily or divided BID, up to max of 600 mg. This may be an option for an older child who has pyelonephritis, but is well enough to go home.
Whom should we admit?
The first thing to consider is age. Any infant younger than 2 months should be admitted for a febrile UTI. Their immune systems and physiologic reserve are just not sufficient to localize and fight off infections reliably.
The truth is, for serious bacterial illness like pneumonia, UTI, or severe soft tissue infections, be careful with any infant less than 4-6 months of age.
Of course, the unwell child – whatever his age – he should be admitted. Think about poor feeding, irritability, dehydration – in that case, just go with your gut and call it pyelonephritis, and admit.
What is the age cut-off for a urine culture?
In adults, we think of urine culture only for high-risk populations, such as pregnant women, the immunocompromised, those with renal abnormalities, the neurologically impaired, or the critically ill, to name a few.
In children, it’s a little simpler. Do it for everyone.
Who is everyone? Think of the urine rule of 10s:
10% of young febrile children will have a UTI
10% of UAs will show no evidence of pyuria
Routine urine culture in all children with suspected or confirmed UTI up to about age 10
What do I do then with urine culture results?
From a quality improvement and safety perspective, consider making this a regular assignment to a qualified clinician.
Check once in 24-48 hours to find possible growth of a single uropathogen with at least 50 000 CFU/mL. Look at the record to see that the child is one some antibiotic, or the reason why he may not. Call the family if needed.
A second check at 48-72 hours may be needed to verify speciation and sensitivities.
The culture check, although tedious, is important to catch those small children who did not present with pyuria and who may need antibiotics, or to verify that the right agent is given.
Ok, so your UA is negative…now what?
The culture is cooking, but you are not convinced. Below is the differential diagnosis for common causes of pyuria in children: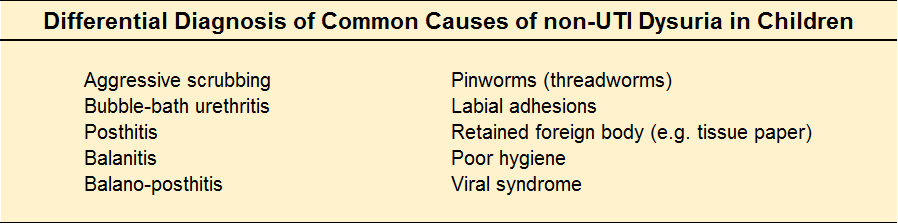
What kind of follow-up should the child get?
The younger the child, the more we worry about missing a decompensation. Encourage the parents to call the child's primary care clinician for a re-check in a few days, and to discuss whether or not further work-up such as imaging is indicated. As always, strict return to ED precautions are helpful.
Who needs imaging?
A more accurate question is: what is an important anomaly to detect?
Vesiculo-ureteral reflux – a loose ureteropelvic junction causes upstream reflux when the bladder constricts.
Uretero-pelvic junction obstruction – in older children or young adults with hematuria, UTI, abdominal mass, or pain. Infants born with UPJ obstruction have congenital hydronephrosis.
Ureterocoele – a cystic mass in the bladder. It is not malignant, but can cause ureteral dilation, and hydronephrosis. Treatment is surgical.
Ectopic ureter – either a duplication of the draining system, or an abnormal connection, such as the epidydimis or cervix.
Posterior urethral valves – occur only in boys, and they are a bit of a misnomer. The most common type of congenital bladder outlet obstruction, posterior urethral valves are just extra folds of membrane in the lumen of the prostatic urethra. Usually ablation by cystoscopy does the trick.
Urachal remnant – a leftover from fetal development, and an abnormal connection between the bladder and the umbilicus. Look for an “always wet” belly button in an infant, or an umbilical mass with pain and fever in an older child.
Imaging of choice as an outpatient?
Renal and bladder ultrasound (RBUS) after the first UTI is recommended (although incompletely followed in practice).
If the RBUS is positive, or with the second UTI, DMSA scan to evaluate possible renal scarring.
So, with all of this testing – are we over doing it?
Like anything, it’s a balance. A few tips to avoid iatrogenia by way of a summary.
If a child over 3 months of age is well, has no comorbidities, has a low grade fever "in the 38s" (38-38.9 °C) without a source, especially if less than 24 hours, you are very safe to do watchful waiting at home.
More to the point, an otherwise well child with an obvious upper respiratory tract infection has a source of his fever.
If your little patient has risk factors for UTI, or you are otherwise concerned, send the UA and send the culture. You can opt out of the culture by middle school in the otherwise healthy child.
And finally, deputize parents to carry the ball from here – the child needs ongoing primary care and his pediatrician may elect to do some screening. Don’t promise or prime them for it – rather, encourage the conversation.
BONUS:
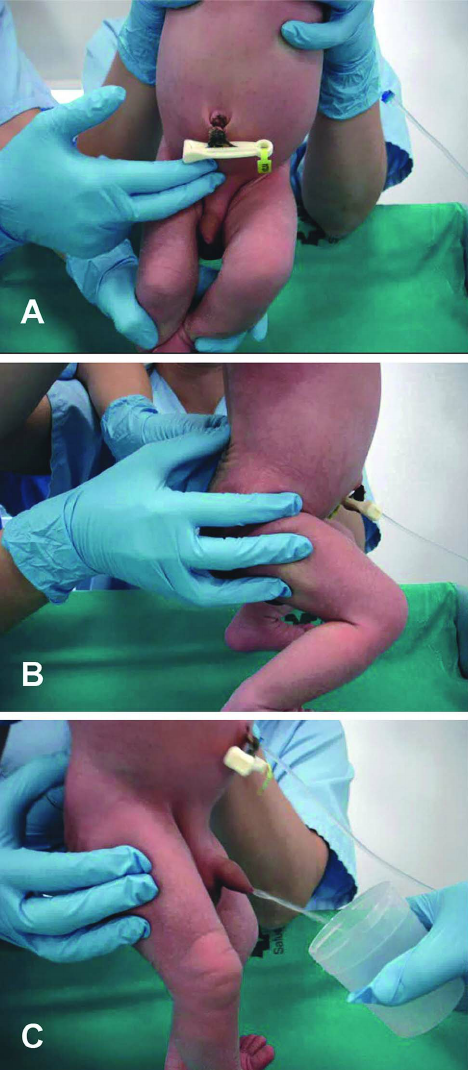
Suprapubic aspiration (details in podcast audio; video below)
BONUS BONUS:
Infant Clean Catch Technique
Step One: feed the baby, wait twenty minutes.
Step Two: clean the genitals with soap and warm water and dry with gauze. Have your sterile urine container open and at the ready.
Step Three: one person holds the baby under his armpits with his legs dangling. The other person gently taps the bladder (100 taps/min), then massages the lower back for 30 seconds.
Step Four: Clean Catch! (can also repeat process)
References
Downs SM. UTI and watchful waiting: the courage to do nothing. Pediatrics. 2014 Mar;133(3):535-6.
This post and podcast are dedicated to Brad Sobolewski, MD, MEd for his innovation and tenacity in all things #FOAMed, #FOAMped, and #MedEd. Thanks, Brad, for your enthusiasm, energy, and for your fantastic PEM Currents and PEM Blog.
Pediatric Urinary Tract Infections
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
N.B.: This month's show notes are a departure from the usual summary. Below is a reprint (with permission) of a soon-to-be released chapter, Horeczko T. "Acute Pain in Children". In Management of Pain and Procedural Sedation in Acute Care. Strayer R, Motov S, Nelson L (eds). 2017. Rather than the customary blog post summary, the full chapter (with links) is provided as a virtual reference.
INTRODUCTION
Pain is multifactorial: it is comprised of physical, psychological, emotional, cultural, and contextual features. In children often the predominant feature may not be initially apparent. Although clinicians may focus on the physical component of pain, much time, energy, and suffering can be saved through a holistic approach. What is the age and developmental stage of the child? How is the child reacting to his condition? What are the circumstances? What is the family or caregiver dynamic?
We rely much on how patients and families interact with us to gauge pain. Assessing and managing children’s pain can be challenging, because they may not exhibit typically recognized signs and symptoms (Srouji 2010). Further, children participate in and absorb their family’s culture and specific personality from a very young age (Finley 2009). Knowing the context of the episode may help. For example, a very anxious caregiver can easily transmit his or her anxiety to the child, which may either inhibit or amplify presentation of symptoms (Bearden 2012).
The guiding principles in pediatric pain assessment and management are: know the child; know the family; and know the physiology. Children have long suffered from an under-treatment of their pain, due both to our incomplete acknowledgement of their pain and our fear of treatment (Howard 2003). As the pendulum on pain management swings one way or the other, do not let your pediatric patient get knocked by the wayside. Take a thoughtful approach: know the signs and symptoms, and aggressively treat and reassess.
ASSESSMENT
Each stage of development offers a unique framework to the child’s signs and symptoms of pain. In pre-verbal children, use your observational skills in addition to the parent’s report of behavior. Verbal children can self-report; younger children require pictorial descriptions, while older children and adolescents may use standard adult scales. In all ages, ask open-ended questions and allow the child to report and speak for himself whenever possible.
Neonates
Neonates are a unique group in pain assessment. The neonate (birth to one month of age) has not yet acquired social expression of pain, and his nascent nervous system is only now learning to process it. Do not expect typical pain behaviors in neonates. Facial grimacing is a weak indicator of pain in neonates (Liebelt 2000). When this behavior is present, look for a furrowed brow, eyes squeezed shut, and a vertically open mouth. Tachycardia, tachypnea, and a change in behavior can be indicators not only to the presence of pain, but possibly to its etiology as well.
Neonatal observational scales have been validated in the intensive care and post-operative settings; ED-specific quantitative scales are lacking. CRIES is a 10-point scale, using a physiologic basis similar to APGAR: Crying; Requires increased oxygen administration (distress and breath-holding); Increased vital signs; Expression; and Sleeplessness (Krechel 1995). CRIES (Table 1) was validated for post-operative patients; to adapt its use for the ED, the most conservative approach is to substitute “preoperative baseline” with normal range for age. Although the numerical values of CRIES have not been validated to date in the ED, the clinician may find the domains included in CRIES to be a useful cognitive construct in assessing neonatal pain.
Neonatal pain pathways are particularly plastic; prompt assessment of and increased alertness to neonatal pain may help to mitigate long-lived pain sensitivity and hyperalgesia (Taddio 2002). In other words, treat the neonate’s pain seriously, as you may save him long-term pain sequelae in the future.
Infants and Toddlers
This group will begin to exhibit more reproducible, reliable signs and symptoms of pain.
For infants of less than one year of age, the Neonatal Infant Pain Scale (NIPS) uses observational and physiologic parameters to detect pain (Table 2). A score of 0-2 indicates no pain present. A score of 3-4 indicates mild to moderate pain; non-pharmacologic techniques may be tried first. A score of 5 or greater indicates severe pain; some pharmacologic intervention is indicated (Lawrence 1993).
For children greater than one year who are preverbal, a well performing scale is the FLACC score: Face, Legs, Activity, Cry, Consolability (Table 3).
Contextual and caregiver features predominate in this group. Frequent reassessments are helpful, as the initial trepidation and fright in triage may not accurately reflect the child’s overall pain status.
Preschool and School-age children
Increasing language development offers the hope of more information to the clinician, but be careful not to ask leading questions. Do not jump directly to “does this hurt?”. Preschoolers will say ‘yes’ to anything, in an attempt to please you. School-age children may passively affirm your “statement”, if only to validate their human need for care or attention. Start with some ice-breaking banter, lay down the foundations for rapport, and then ask open-ended questions. Be careful not to allow the caregiver to “instruct” the child to tell you where it hurts, how much, how often, etc. Rather, engage the parents by asking them what behavior they have noticed. Eliciting history from both the child and the parent will go a long way in constructing a richer picture of the etiology and severity of the pain, and will help to build rapport and trust.
The Baker-Wong FACES Pain Rating scale (Figure 1) was developed with feedback from children and has been validated for use in those 3 years of age and older (Keck 1996, Tomlinson 2010).
Adolescents
Adolescents vary in their development, maturity, and coping mechanisms. You may see a mixture of childhood and adult behaviors in the same patient; e.g. he may be initially stoic or evades questioning, then later exhibits pseudo-inconsolability. Do what you can to see the visit from the adolescent’s perspective, and actively transmit your concern and intention to help – many will respond to a warm, open, non-judgemental, and helpful attitude. The overly “tough” adolescent is likely secretly fearful, and the “dramatic” adolescent may simply be very anxious. Take a moment to gauge the background behind the presentation.
You may use the typical adult scale of 0 (no pain) to 10 (worst pain), or the Faces Pain Scale–Revised (FPS-R). The FPS-R uses more neutral and realistic faces and, unlike the Wong Baker scale, does not use smiling or crying faces to anchor the extremes of pain (Tsze 2013).
PAIN PHYSIOLOGY
Pain includes two major components: generation and perception. Generation of pain involves the actual propagation of painful stimuli, either through nociceptive pain or neuropathic pain. Nociceptive pain arises from free nerve endings responding to tissue damage or inflammation.
Nociceptive pain follows a specific sequence: transduction (an action potential triggered by chemical mediators in the tissue, such as prostaglandins, histamine, bradykinin, and substance P); transmission (the movement of the action potential signal along the nerve fibers to the spinal cord); perception (the impulse travels up the spinothalamic tract to the thalamus and midbrain, where input is splayed out to the limbic system, somatosensory cortex, and parietal and frontal lobes); and modulation (the midbrain enlists endorphins, enkephalins, dynorphin, and serotonin to mitigate pain) (Pasero 2011). As clinicians we can target specific “stations” along the pain route to target the signal more effectively.
Simple actions such as ice, elevation, local anesthetics, or splinting help in pain transduction. Various standard oral, intranasal, or IV analgesics may help with pain’s transmission. Non-pharmacologic techniques such as distraction, re-framing, and others can help with pain perception. The sum of these efforts encourage pain modulation.
A phenomenon separate from nociceptive pain is neuropathic pain, the abnormal processing of pain stimuli. It is a dysregulated, chaotic process that is difficult to manage in any setting. Separating nociceptive from neuropathic symptoms may help to select specific pain treatments and to clarify treatment goals and expectations.
Neonates
Neonates are exquisitely sensitive to many analgesics. Hepatic enzymes are immature and exhibit decreased clearance and prolonged circulating levels of the drug administered. Once the pain is controlled, less frequent administration of medications, with frequent reassessments, are indicated.
The neonate’s vital organs (brain, heart, viscera) make up a larger proportion of his body mass than do muscle and fat. That is to say, the volume of distribution is unique in a neonate. Water-soluble drugs (e.g. morphine) reach these highly perfused vital organs quickly; relatively small overdosing will have rapid and exaggerated central nervous system and cardiac effects. The neonate’s small fat stores and muscle mass limit the volume of distribution of lipophilic medications (e.g. fentanyl, meperidine), also making them more available to the central nervous system, and therefore more potent. Other factors that predispose neonates to accidental analgesic overdose are their decreased concentrations of albumin and other plasma proteins, causing a higher proportion of unbound drug. Renal clearance is also decreased in the first few months of life.
Clinical note: in the ED, neonates often require analgesia for procedures more than for injury. Non-pharmacologic techniques predominate (see below). Make liberal use of local anesthetics such as eutectic mixture of local anesthetics (EMLA; for intact skin, e.g. IV access, lumbar puncture) and lidocaine-epinephrine-tetracaine gel (LET; for superficial open skin and soft tissue application). Oral sucrose (30%) solutions (administered either with a small-volume syringe or pacifier frequently dipped in solution) are effective for minor procedures (Harrison 2010, Stevens 2013) via the release of dopamine and through distraction by mechanical means. Neonates with severe pain may be managed with parenteral analgesics, on a monitor, and with caution.
Infants and Toddlers
With increasing body mass comprised of fat stores in conjunction with an increase in metabolism, this group will require a different approach than the neonate. For many medications, these children will have a greater weight-normalized clearance than adults (Berde 2002). They will often require more frequent dosing. Infants and toddlers have a larger functioning liver mass per kilogram of body weight, with implications for medications cleared by cytochrome p-450.
Clinical note: some drugs, such as benzodiazepines, will have both a per-kilogram dosing as well as an age-specific modification. When giving analgesics or anxiolytics to young children, always consult a reference for proper dosing and frequency.
School-age children and Adolescents
This group retains some hyper-metabolic features of younger children, but the dose-effect relationship is more linear and transparent. Physiologic clearance is improved, and from a physical standpoint, these are typically lower-risk children. From a psychological standpoint, this group may need more non-pharmacologic consideration and support to modulate pain optimally.
NON-PHARMACOLOGIC TREATMENT
The first line of treatment in all pain management is non-pharmacopeia (Horeczko 2016). Not only is this the safest of all techniques, but often the most effective. Some are simple comfort measures such as splinting (fracture or sprain), applying cold (acute soft tissue injury) or heat (non-traumatic, non-specific pain), or other targeted non-pharmacology.
Many a pain control regimen is sabotaged without consideration of non-pharmacologic techniques, which may augment, or at times replace, analgesics. Think of non-pharmacopoeia as your “base coat” or “primer” before applying additional coats of analgesic treatment. With the right base coat foundation, you have a better chance of painting a patient’s symptoms a more tolerable and long-lasting new color.
A tailored approach based on age will allow the practitioner to employ a child’s developmental strengths and avoid the frustration that results in asking the child to do what he is not capable of doing. A brief review of Piaget’s stages of development will help to meet the child at his developmental stage for best effect (Piaget 1928, Sheppard 1977) during acute painful presentations and minor procedures.
Sensorimotor stage (from birth to age 2): Children use the five senses and movement to explore the world. They are egocentric: they cannot see the world from another’s viewpoint. At 6 to 9 months, object permanence is established: understanding that objects (or people) exist even without seeing them.
Preoperational stage (from ages 2 to 7): Children learn to use language. Magical thinking predominates. They do not understand rational or logical thinking.
Concrete operational stage (from age 7 to early adolescence): Children can use logic, but in a very straightforward, concrete manner (they do well with simple examples). By this stage, they move from egocentrism to understanding another point of view. N.B. Some children (and adults) never completely clear this stage.
Formal operational stage (early adolescence to adult): children are capable of abstract thinking, rationalizing, and logical thinking.
It is important to assess the child’s general level of development when preparing and guiding him through the minor procedure or distracting him until his pain is controlled. It is not uncommon for acutely ill or injured to regress temporarily in their behavior (not their development) as a coping mechanism.
Neonate and Infant (0-12 months)
Involve the parent, and have the parent visible to the child at all times if possible. Make advances slowly, in a non-threatening manner; limit the number of staff in the room. Use soothing sensory measures: speak softly, offer a pacifier, and stroke the skin softly. Swaddle the infant and encourage the parent to comfort him during and after the procedure. Engage their developing sensorimotor skills to distract them.
Toddler to Preschooler (1-5 years)
Use the same techniques as for the infant, and add descriptions of what he will see, hear, and feel; you can use a doll or toy to demonstrate the procedure. Use simple, direct language, and give calm, firm directions, one at a time. Explain what you are doing just before doing it (do not allow too much time for fear or anxiety to take root). Offer choices when appropriate; ignore temper tantrums. Distraction techniques include storytelling, bright and flashy toys, blowing bubbles, pinwheels, or having another staff member play peek-a-boo across the room. The ubiquitous smart phone with videos or games can be mesmerizing at this age.
School age (6-12 years)
Explain procedures using simple language and (briefly) the reason (understanding of bodily functions is vague in this age group). Allow the child to ask questions, and involve him when possible or appropriate. Distraction techniques may include electronic games, videos, guided imagery, and participation in the minor procedure as appropriate.
Adolescent (13 and up)
Use the same techniques for the school age child, but can add detail. Encourage questioning. Impose as few restrictions as possible – be flexible. Expect more regression to childish coping mechanisms in this age group. Distraction techniques include electronic games, video, guided imagery, muscle relaxation-meditation, and music (especially the adolescent’s own music, if available).
APPLIED PHARMACOLOGY
No amount of knowledge of the above physiology, pharmacology, or developmental theory will help your little patient in pain without a well constructed and enacted plan. Aggressively search out and treat your pediatric patient’s presence and source of pain. Frequent reassessments are important to ensure that breakthrough pain treatment is achieved, when re-administration is indicated, or when a change of plan is necessary. This is the time to involve the parents or caregivers to let them know what the next steps are, and what to expect.
Start with the least invasive modality and progress as needed. After non-pharmacologic treatments such as splinting, ice, elevation, distraction, and guided imagery, have an escalation of care in mind (Figure 2).
From a pharmacologic perspective, various options are available. Your pain management plan will differ depending on whether a painful procedure is performed in the ED (Table 4). Once pain is addressed, create a plan to keep it managed. Consider the trajectory of illness and the expected time frame of the painful episode. Include practicalities such as how well the pain may be controlled as an outpatient. Poorly controlled pediatric pain is more often managed as an inpatient than the same condition in an adult. Speak frankly with the parents about what drug is indicated for what type of pain and that treatment goals typically do not include absence of all pain, but function in face of the pain, in anticipation for clinical improvement.
A special note on codeine: Tylenol with codeine (“T3”) has never been a very effective pain medication, as up to 10% of patients lack enzymatic activity to metabolize it into morphine, its active form (Crews 2014). New evidence is emerging on the erratic and unpredictable individual metabolism of codeine. Some children are ultra-rapid-metabolizers of codeine to morphine, causing a rapid “bolus” of the available drug, with respiratory depression and death in some cases (Ciszkowski 2009, Racoosin 2013). Author’s advice: take codeine off your formulary.
COMMON SCENARIOS
Head and neck pain
Most common non-traumatic head and neck complaints can be managed non-pharmacologically (e.g. headache: improved hydration, sleep, stress, nutrition) or with PO medications, such as NSAIDs. The anti-inflammatory nature of ibuprofen (10 mg/kg PO q 4-6 h prn, up to adult dose) for example, will treat the cause as well as the symptoms of ear pain, sore throat, and muscular pain. Ibuprofen may be more effective than acetaminophen (paracetamol) for odontogenic pain (Bailey 2013). For most applications, acetaminophen may be as effective; however, the combination of both NSAIDs is not likely to be more effective than either agent individually (Merry 2013).
True migraine headache may be treated with all of the above, and rescue therapy may include prochlorperamide (0.15 mg/kg IV, up to 10 mg ) (Brousseau 2004), often given with diphenhydramine (1 mg/kg PO or IV, up to 50 mg) and IV fluids. Ketoralac (0.5 mg/kg IV, up to 10 mg) may be substituted for ibuprofen (Paniyot 2016). Other specific therapies may be considered, although evidence for them varies.
Chest pain
After ruling out important pulmonary (e.g. the under-recognized spontaneous pneumothorax) and cardiac (e.g. pericarditis, myocarditis) etiologies, many chest complaints are amenable to NSAIDs. There is often a large component of anxiety in the child and/or parents in chest pain; no amount of medication will assuage them without addressing their concerns as well.
Abdominal pain
Abdominal pain in children is challenging, as it is common, often benign, but may be disastrous if the etiology is missed. For mild pain, consider acetaminophen as indicated (15 mg/kg/dose q 4-6 h prn, up to 650 mg). The oral route is preferred, but intravenous acetaminophen is an option for patients unable to tolerate PO, or for those in whom the per rectum (PR) route is contraindicated (e.g. neutropenia) (Babl 2011, Dokko 2014). For children with moderate to severe abdominal pain in whom a nil per os (NPO) status is ideal, consider rehydration/volume repletion, and small, frequent aliquots of a narcotic agent. Surgical pain is not “erased” by opioids (Thomas 2003, Poonai 2014); treating pain improves specificity to certain surgical emergencies with retained diagnostic accuracy (Manterola 2007). If there is inter-departmental concern about prolonged effects, sedation, limitation in the physical exam, or there is a need to “see if the pain will come back”, you may opt to use fentanyl initially for its shorter half-life. More frequent re-assessments may help the surgical team in its deliberations. Transition quickly to a longer-acting opioid as soon as possible.
Long-bone injuries
Fracture pain should be addressed immediately with splinting and analgesia. Oral, intranasal, and intravenous routes are all acceptable, depending on the severity of the injury and symptoms.
Intranasal (IN) medications offer the advantage of a fast onset for moderate-to-severe pain (Graudins 2015), either as monotherapy or as a bridge to parenteral treatment (Table 4). The ideal volume of IN medication is 0.25 mL/naris, with a maximum of 1 mL/naris. Common concentrations of fentanyl limit its mg/kg use to the school-aged child; intranasal ketamine may be used for pain (i.e. sub-dissociative dose) up to adult weight.
Long-bone injuries are a good opportunity to employ a speedy modality that requires little technical skill in administration: nebulized fentanyl. Clinically significant improvement in pain scales are achieved with 3 mcg/kg/dose of fentanyl administered via standard nebulizer in children 3 years of age or older (Miner 2007, Furyk 2009). Nebulized fentanyl is a rapid, non-invasive alternative to the IN route for older children, adolescents, or adults, in whom the volume of IN medication would exceed the recommended per naris volume (Deaton 2015).
Consider an aggressive, multi-modal approach to control symptom up front. For example, for a simple forearm fracture, you may opt to give an oral opioid, perform a hematoma block, and offer inhaled nitrous oxide for reduction, rather than a formal intravenous procedural sedation (Luhmann 2006).
Ultrasound-guided peripheral nerve blocks are a good pain control adjunct, after initial treatment, and in communication with referring consultants (Ganesh 2009, Suresh 2014).
Skin and Soft tissue
Skin and soft tissue injuries or abscesses often require solid non-pharmacopoeia in addition to local anesthetics. For IV cannulation, consider EMLA if the patient is stable and a minor delay is acceptable.
Topical ethyl chloride vapo-coolant offers transient pain relief due to rapid cooling and may be used just prior to an IV start (Farion 2008). Try this: engage your young child’s imagination to distract him and say, “have you ever held a snow ball? You are in luck – it’s just like that – here, do you feel it?”.
Vibratory adjuncts such as the “BUZZY” bee can be placed near the IV cannulation site to provide mechanical and cognitive distraction (Moadad 2016).
Needleless lidocaine injectors may facilitate IV placement without obscuring the target vein (Spanos 2008, Lunoe 2015). The medication is propelled into the dermis by a CO2 cartridge that makes a loud popping sound; try this to alleviate anxiety, just before using it: “your skin looks thirsty – it needs a drink – there you are!”.
As with any minor procedure, when you tell the child what you are doing, be sure to do it right away. Do not delay or build suspense.
Lidocaine-epinephrine-tetracaine gel (LET) is used for open or mucosal wounds. Apply as soon as possible in the visit. The goal of LET is to pretreat the wound to allow for a painless administration of injectable anesthetic. A common practice to apply LET two or three times at 15-minute intervals for deeper anesthesia, in an attempt to avoid injection altogether. Researchers are currently working to offer an evidence base to this anecdotal practice.
Pediatric burns should be assessed carefully and treated aggressively. Submersion of the affected extremity in room-temperature water (if possible) or applying room-temperature saline-soaked gauze will both thwart ongoing thermal damage, soothe the wound, and provide foundational first-aid. Minor burns can be treated topically and with oral medications. Major burns require IN, IM, or IV analgesics with morphine. Treatment may escalate to ketamine (Gandhi 2010), in analgesic or dissociative dosing, depending on the context. Post-traumatic disorders are common in burns; effective pain management is ever-more important in these cases.
SPECIFIC SCENARIOS
The child with chronic medical problems
Children with acute exacerbations of their chronic pain or episodic painful crises require special attention. Some examples of children with recurring pain are those suffering from sickle cell disease, juvenile idiopathic arthritis, complex regional pain syndrome, and cancer. Find out whether these symptoms and circumstances are typical for them, and what regimen has helped in the past. Previous unpleasant experiences may prime these children with amplified anxiety and perception of pain (Cornelissen 2014). Target the disease process and do your best to show the patient and his family you understand his condition and needs.
An equally challenging scenario is the child with chronic pain. Treat the entire patient with a multimodal approach. Limit opioids as possible. As an opioid-sparing strategy or as rescue therapy, consider sub-dissociative ketamine, especially for conditions such as sickle cell crisis, complex regional pain syndrome, autoimmune disorders, or chronic pain due to sub-acute trauma (Sheehy 2015). Intranasal ketamine may be used for sub-dissociative pain control at 0.5 – 1 mg/kg (Andolfatto 2013, Yeaman 2013). Intravenous infusions of ketamine at 0.1 – 0.3 mg/kg/h may be initiated in the ED and continued 4 – 8 h/d, up to a maximum of 16 h total in 3 consecutive days (Sheehy 2015). In vaso-occlusive episodes, dexmedetomidine has been shown to be an effective adjunct for severe pain poorly responsive to opioids and/or ketamine (Sheehy 2015b).
The child with cognitive impairment
Children with cognitive impairment such as those with various genetic or metabolic syndromes, or primary neurologic conditions such as some with cerebral palsy are a challenge to assess and treat properly. These children not only cannot explain their symptoms, but they also have atypical expressions of pain. Pain responses in severely intellectually disabled children include a full-blown smile (which may or may not accompany inappropriate laughter), stiffening, and non-cooperation (Hadden 2002). Other observed behaviors include the freezing phenomenon, in which the child acutely feels the pain, and he abruptly pauses without moving his face for several seconds. Look also for episodes of unexplained pallor, diaphoresis, breath-holding, and shrill vocalizations. The FLACC has been revised (r-FLACC) for children with cognitive impairment and appears to be reliable for acute care (Malviya 2006).
The most distressing and perplexing presentation is the parent who brings his or her child with cognitive impairment for “fussiness”, “irritability”, or “I think he’s in pain”. Often, this is after significant investigations have been performed, sometimes repeatedly. Poorly controlled spasticity is an often under-appreciated cause of unexplained pain; treat not with opioids, but with GABA-receptor agonists, such as baclofen or benzodiazepines.
Take special precautions in the administration of opioids or benzodiazepines in children with metabolic disorders (e.g. mitochondrial disease) or various syndromes (e.g. Trisomy 21). They may have a disproportionate reaction to the medication. Start with a low dose in these children and reassess frequently, titrating in small aliquots as needed.
After careful, meticulous investigation in the ED to rule out occult infection, trauma, electrolyte imbalance, or surgical causes, the child with cognitive impairment who continues to be symptomatic despite ED treatment may be admitted for observation. However, in some cases, the addition of gabapentin to the typical regimen has been shown to manage unexplained irritability in these children (Hauer 2007) by treating visceral hyperalgesia.
Multi-trauma
The child with multi-trauma is in need of meticulous critical care. Frequent assessments of pain analgesic response (typically via the intravenous route) are necessary to gauge the child’s trajectory. Unexplained tachycardia may be the early signs of shock. Without controlling the child’s pain, it is difficult to distinguish the extreme tachycardia from pain or from blood loss. If intubated, control the pain first with a fentanyl drip, then use a sedative in addition as needed to keep him comfortable.
The child under palliative care
Children undergoing palliative care require a multidisciplinary approach. This includes engaging the patient’s car team as well as “treating” members of the patient’s family. Examples include the natural course of devastating chromosomal, neurologic, and other congenital conditions; terminal cancer; and trauma, among others (Michelson 2007). Family dynamics and family members’ needs are often overlooked; the family as a whole must be considered. Focus on the productive and beneficial treatments that can be offered. Treat pain promptly, but speak with the parents about end-of-life goals as early as possible, as any analgesic or sedative may have an untoward effect. You do not want to be caught in the position of potentially precipitously providing cardiopulmonary resuscitation in a child undergoing palliative care, because of a lack of understanding of how increasingly large doses of pain medications can affect breathing and circulation (AAP 2000).
Children with ongoing opioid requirements may present not so much with an exacerbation of their chronic pain, but a complication of its treatment. Identify, assess and aggressively treat constipation, nausea and vomiting, pruritus, and urinary retention (Friedrichsdorf 2007); treating side-effects of pain management may be just as important for quality of life as treating the pain itself.
PEARLS AND PITFALLS IN PEDIATRIC PAIN
Allow the child to speak for himself whenever possible. After acknowledging the parent’s input, perhaps try “I want to make sure I understand how the pain is for you. Tell me more.”
Engage parents and communicate the plan to them. Elicit their expectations, and give them of preview of what to expect in the ED.
Opioids are meant for pain caused by acute tissue injury, for the briefest period of time feasible. Older school-aged children and adolescents are increasingly at risk for opioid dependence and addiction.
Premature infants present a challenge in pain control. Their pain is under-recognized, as they often display atypical responses to painful stimuli. Treatment is equally difficult, as they are particularly sensitive to analgesia-sedation. This is important, as this group is even more likely to undergo painful procedures due to their higher-risk status.
Give detailed advice on how to manage pain at home. Set expectations. Let them know you understand and will help them through your good advice that will carry them through this difficult time. Patients and families often just need a plan. Map it out clearly.
SUMMARY
- In pediatric acute pain, know the child; know the family; and know the physiology.
- Use your observational skills enhanced with collateral information to assess and reassess for pain in children.
- Treat pediatric pain well and often. Failure to address the child’s pain has long-lasting consequences.
- Non-pharmacologic treatments for all, pharmacologic treatments for many. A multi-modal approach is the most effective.
- Neonates, infants and toddlers, and school-aged children and adolescents exhibit specific physiology in expression of pain and in response to treatment. Tailor your regimen to your young patient’s physiologic pitfalls and needs.
References
Howard RF. Current status of pain management in children. JAMA. 2003 Nov 12;290(18):2464-9.
Pasero C, McCaffery M. Pain Assessment and Pharmacologic Management. St. Louis, Mo: Mosby; 2011.
Piaget J. Judgment and reasoning in the child. Harcourt & Brace. Oxford, England. 1928.
This post and podcast are dedicated to Sergey M. Motov, MD, FAAEM, for his integrity, hard-won expertise, humility, and innovation. Thank you for making us better doctors, Sergey, and for getting us ever closer to a pain-free ED.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
"By the pricking of my thumbs,
Something wheezing this way comes."
-- Witches in Macbeth, with apologies to William Shakespeare
"Bronchiolitis is like a pneumonia you can’t treat.
We support, while the patient heals."
-- Coach, still apologetic to the Bard
The Who
The U.S. definition is for children less than two years of age, while the European committee includes infants less than one year of age.
This is important: toddlerhood brings with it other conditions that mimic bronchiolitis – the first-time wheeze in a toddler may be his reactive airway response to a viral illness and not necessarily bronchiolitis.
The What
The classic clinical presentation of bronchiolitis starts just like any other upper respiratory tract infection: with nasal discharge and cough, for the first 1-2 days. Only about 1/3 of infants will have a low-grade fever, usually less than 39°C. We may see the child in the ED at this point and not appreciate any respiratory distress – this is why precautionary advice is so important in general.
Then, lower respiratory symptoms come: increased work of breathing, persistent cough, tachypnea, retractions, belly breathing, grunting, and nasal flaring. Once lower respiratory symptoms are present, like increased work of breathing, they typically peak at day 3. This may help to make decisions or counsel parents depending on when the child presents and how symptomatic he is.
You’ll hear fine crackles and wheeze. A typical finding in bronchiolitis is a minute-to-minute variation in clinical findings – one moment the child could look like he’s drowning in his secretions, and the next minute almost recovered. This has to do with the dynamic nature of the secretion, plugging, obstruction, coughing, dislodgement, and re-plugging.
The Why
Respiratory syncytial virus is the culprit in up to 90% of cases of bronchiolitis. The reason RSV is so nasty is the immune response to the virus: it binds to epithelial cells, replicates, and the submucosa becomes edematous and hypersecretes mucus. RSV causes the host epithelia and lymphocytes to go into a frenzy – viral fusion proteins turn the membranes into a sticky goop – cells fuse into other cells, and you have a pile-on of multinucleated dysfunction. This mucosal chaos causes epithelial necrosis, destruction of cilia, mucus plugs, bronchiolar obstruction, air trapping, and lobar collapse.
High-Risk Groups
Watch out especially for young infants, so those less than 3 months of age. Apnea may be the presenting symptom of RSV.
Premature infants, especially those less than 32 weeks’ gestation are at high risk for deterioration. The critical time is 48 weeks post-conceptional age.
Other populations at high-risk for deterioration: congenital heart disease, pulmonary disease, neuromuscular disorders, metabolic disorders.
Guiding Principles
In the full term child, greater than one month, and otherwise healthy (no cardiac, pulmonary, neuromuscular, or metabolic disease), we can look to three simple criteria for home discharge.
If the otherwise healthy child one month and older is:
Euvolemic
Not hypoxic
Well appearing
He can likely go home.
The How
Below is a list of modalities, treatments, and the evidence and/or recommendations for or against:
Chest Radiograph
Usually not necessary, unless the diagnosis is uncertain, or if the child is critically ill.
Factors that are predictive of a definite infiltrate are: significant hypoxia (< 92%), grunting, focal crackles, or high fever (> 39°C).
Ultrasound
Not ready for prime time. Two small studies, one by Caiulo et al in the European J or Pediatrics and one by Basile et al. in the BMC Pediatrics that show some preliminary data, but not enough to change practice yet.
Viral Testing
Qualitative PCR gives you a yes or no question – one that you’ve already answered. It is not recommended for routine use. PCR may be positive post-infection for several weeks later (details in audio).
Quantitative PCR measures viral load; an increased quantitative viral load is associated with increased length of stay, use of respiratory support, need for intensive care, and recurrent wheezing. However, also not recommended for routine use.
There is one instance in which viral testing in bronchiolitis can be helpful – in babies less than a month of life, the presence of RSV virus is associated with apnea.
Blood or Urine Testing
Routine testing of blood or urine is not recommended for children with bronchiolitis. Levine et al in Pediatrics found an extremely low risk of serious bacterial illness in young febrile infants with RSV.
The main thing is not to give in to anchoring bias here. If an infant of 3 months of age or older has a clear source for his low-grade fever – and that is his bronchiolitis – then you have a source, and very rarely do you need to go looking any further. He’s showing you the viral waterfall from his nose, and his increased work of breathing. It’s not going to be in his urine.
Bronchodilators!
Should we use bronchodilators in bronchiolitis? It seems lately that this is a loaded question – with strong feelings on either side amongst colleagues. The short answer is that the American Academy of Pediatrics, the UK’s National Institute for Health and Care Excellence, as well as the Canadian Pediatric Society currently recommend against them. However, in continental Europe and Australia, the language is softened to “not routinely recommended”.
Pros and Cons in Audio; the 2006 AAP Guidelines and the 2014 AAP Guidelines use same data to come to divergent recommendations.
Steroids
There is no role for steroids in the treatment of bronchiolitis, even in those with a family or personal history of atopy.
Nebulized Hypertonic Saline
May show some benefit in admitted patients, after repeated treatments; no data to support its use in ED patients (no immediate effect).
Nebulized Epinephrine
One randomized controlled double blinded study in eight centers in Norway published in the NEJM showed no benefit to nebulized epinephrine over nebulized saline. Again, probably asking too much of one single intervention.
The Cochrane review found 19 studies that included a total of 2256 children with acute bronchiolitis treated with nebulized epinephrine. There were no differences in length of hospital stay between the placebo and treatment groups, and so they concluded that for inpatients, nebulized epinephrine is not worth the hassle. However – and this may just be an artifact of meta-analysis – there may be some benefit to outpatients. One study of combined high-dose steroid and epinephrine therapy was not statistically significant when other factors were controlled, but Cochrane concluded that nebulized epinephrine itself may be helpful for outpatients. It won’t affect the overall disease time course, but it may make them feel better enough to go home from the ED and continue observation there.
High-Flow Nasal Cannula Oxygen
High-flow oxygen via nasal cannula requires specialized equipment and delivers humidified oxygen at 1-2 L/g/min. In addition to oxygenation, high flow nasal cannula also likely offers some low-grade positive end-expiratory pressure, which may help with alveolar recruitment. The evidence for its use is based on observational studies, which have found improved respiratory parameters and reduced rates of intubation. Nasal CPAP also has some promising properties in the right clinical setting.
Antibiotics
Not recommended. When bronchiolitis is from a clear viral source, the risk of accompanying bacteremia is less than 1%. A meta-analysis of randomized clinical trials found that antibiotics in bronchiolitis did not improve duration of symptoms, length of hospital stay, need for oxygen therapy, or hospital admission.
Summary: The Good, the Bad, and the Ugly
The Good
Nasal suction and hydration are your best allies. You may elect to give a bronchodilator as a trial once and reexamine, if you’re a bronchodilating believer.
The Bad
Steroids, antibiotics, and a blind obeying of the guidelines. Weigh the risks and benefits of every intervention, including hospitalization – it’s not always a benign thing.
The Ugly
Take a moment to assess the child and make a clinical diagnosis of bronchiolitis, after you’ve excluded cardiac disease, anatomic anomalies, and foreign body aspiration. Wheezing without upper respiratory symptoms is not viral, and it is not bronchiolitis.
When all else fails, remember: in the otherwise healthy, term infant greater than a month of age, if he is well appearing, euvolemic, and not hypoxic, he will often do well with good precautionary advice and supportive care at home. Every thing else: be skeptical, be thorough, and above all, be careful.
Florin TA, Plint AC, Zorc JJ. Viral bronchiolitis. Lancet. 2016 Aug 20. [Epub ahead of print]
Meissner HC. Viral Bronchiolitis in Children. N Engl J Med. 2016 Jan 7;374(1):62-72.
This post and podcast are dedicated to Linda Girgis MD, FAAFP, for her authenticity, innovation, and clear and honest voice on the the frontlines. Thank you, Dr Linda.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
Johnny has fallen on an outstretched hand, and comes to you with a swollen, painful elbow.
Position of comfort, analgesia, xrays, and now what?
What am I seeing -- or not seeing -- here?
First a refresher on radiographic anatomy of the elbow --
Images courtesy of Radioglypics (Open Access Radiology Education). Used with permission.
Now that we have our adult anatomy reviewed, let's go through the development of the elbow in a child.
We are all born with primary ossification centers -- the basic shapes of our long bones. Secondary ossification centers then develop around the ends of our long bones, and make interpretation of films in the context of suspected injury difficult.
Elbow Interpretation Roadmap: CRITOE
More pragmatic and utilitarian than a prosaic mnemonic, CRITOE helps us to remember the order of ossification of the pediatric elbow.
Although children develop at different rates, the order of ossification is programmed into us. Images courtesy of Radiopaedia.
Capitellum
By age one, the capitellum ossifies. On the AP view, imagine a little white oval balloon floating in the darkness between the radius and the humerus.
Radial Head
By age three, the capitellum gets another little balloon to join the party. The radial head is a bony little balloon that floats just above the floor. If you see both little balloons floating on either ends of the space between the humerus and the radius – you know this child is about three years old.
Internal Epicondyle
By the age of five, the capitellum and radial head are no longer little floating balloons, but now taking on shapes that resemble what they will look like as an adult. By age five, you’ve grown out of balloons, and have moved on to Frisbees. The internal epicondyle (meaning the medial epicondyle) starts to ossify by age five – a little bony Frisbee.
Trochlea
By age seven, another little Frisbee flies around. On the AP view, the trochlea is superimposed on the humerus – if you look at the distal medial humerus, you’ll see the trochlea like a little oval Frisbee taking shape (see combined film below).
Olecranon
By age nine, the olecranon of the ulna is ossifying. In a nine year old, you’ll see a capitellum, radial head, internal epicondyle, trochlea, and olecranon.
External Epidondyle
By age 11, you start to ossify your external epicondyle (lateral epicondyle).
Pediatric Elbow Films: Putting It All Together
Watch this dynamic video by Dr Jeremy Jones from Radiopaedia:
Fracture Saviors: Fat Pads and Drawn Lines
These three things can save us: fat pads, the anterior humeral line, and the radiocapitellar line. Non-annotated images courtesy of Heidi Nunn.
Normal anterior fat pad
Sail sign: billowing hypodensity, indicating blood; sometimes the only (indirect) sign of an elbow fracture
Posterior fat pad: always pathologic
Radiocapitellar Line: anterior humeral line bisects the capitellum
Baumann’s angle (carrying angle): Normal is 70 to 75 degrees. A difference between extremities of just 5 degrees or more is abnormal.
Supracondylar fractures: Gartland Classification
Compartment Syndrome
Pain out of proportion to exam, paresthesias, pallor, poikilothermia, pulselessness, and paralysis
The 6 Ps of compartment syndrome are not sensitive in children.
The only thing that may alert you to increasing compartment pressures in children is an increasing need for analgesics.
Volkmann's ischemic contracture
Untreated compartment syndrome results in thrombosis, edema, ischemia, and disabling contracture.
Other Elbow Injuries
(Details in podcast audio)
Lateral Condyle Fracture
Medial Epicondyle Fracture
Radial head and radial neck fractures
Olecranon fractures
Elbow dislocation
Radial head subluxation (nursemaid’s elbow)
Medial epicondylar apophysitis (Little leager’s elbow)
Test your retention: check out this interactive post from the team at Don't Forget the Bubbles.
Key Points and Summary
The most important pediatric elbow injury is the supracondylar fracture. Grade I is minimally displaced and needs a cast; Grade II is displaced, but with the posterior cortex intact; after closed reduction, the child may still need surgery; Grade III fractures all need closed reduction, internal fixation, and close monitoring for compartment syndrome.
CRITOE gives us the order of ossification for the pediatric elbow – capitellum, radial head, internal epicondyle, trochlea, external epicondyle, and olecranon -- typically occurring at year 1, 3, 5, 7, 9, and 11 – remember the order is the most important thing – all ossification centers should be accounted for. Make sure one is not missing – or where one has been “created” traumatically.
If you don't see the obvious fracture, you can be "saved" by the sail sign and/or a posterior fat pad. Also, make sure to look for the anterior humeral line – on the lateral view, a line drawn down the anterior humerus – if it intersects with the middle third of the capitellum, that is normal – it not, suspect a supracondylar fracture.
The radiocapetellar line runs along the radial neck through the radial head and should line up nicely with the capitellum. If not, assume a fracture-dislocation.
Close communication and coordination with the orthopedist will help us to get the right care at the right time – there is some variability with orthopedic practice, so be open to that – we can make out biggest impact by making the right diagnosis, and aggressively treating pain and effectively providing procedural sedation when needed.
References
Bonus! Watch Larry Mellick Reduce a Nursemaid's Elbow!
https://www.youtube.com/watch?v=-0ROu4hCXwQ
This post and podcast are dedicated to Andy Neill, MBBS. Thank you for your humanism and your dogged dedication to connect with the learner and simplify complex concepts. Welcome back, Andy!
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
Blood in the vomit.
Blood in the stool.
Blood in the diaper.
How far do I go in my investigation?
What do I really have to worry about?
The differential diagnosis of GI bleeding in children is broad.
(Here is the complete differential diagnosis)
In the ED, we can simplify by categorizing by age and appearance. 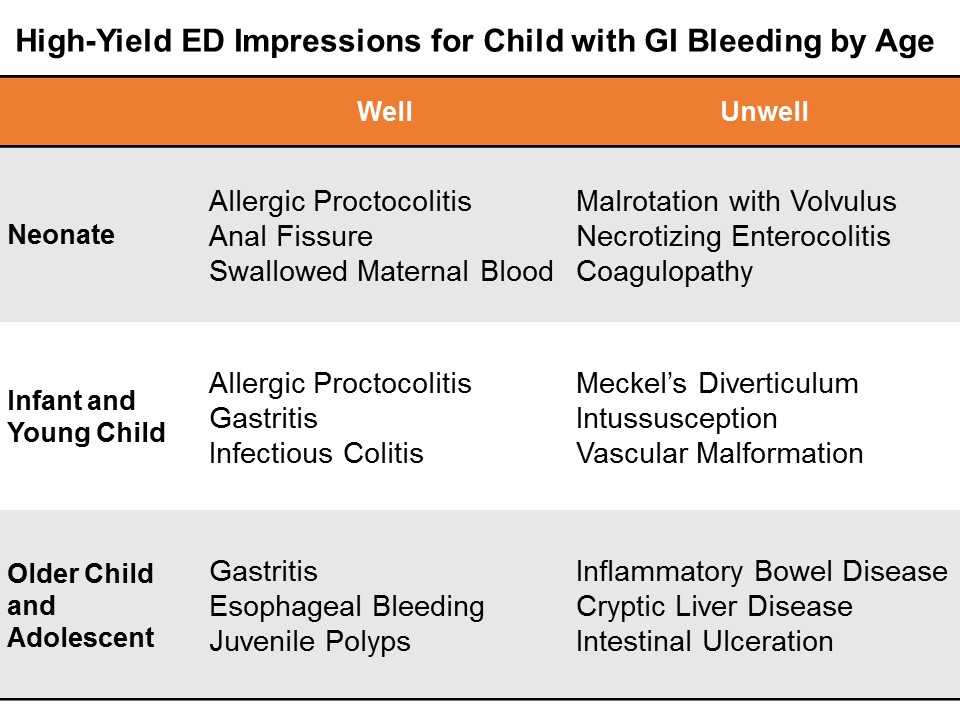
Neonates
GI bleeding in the neonate (less than one month of age) is serious until proven otherwise.
Well appearing?
If this in obvious anal fissure, then no further work-up is necessary. Counsel on proper feeding and follow-up.
Evaluate for potential swallowed maternal blood by examining mother with a chaperone, then perform the Apt test.
Consider allergic proctocolitis if the child is well. Counsel the breastfeeding mother on diet modification. If formula fed, the child should feed through thus until the primary care physician decides whether to start the sticky process of changing up formulas.
If unclear, consider a complete blood count and/or further work-up and admission if unwell.
Ill Appearing?
The three most dangerous diagnoses in the neonate are necrotizing enterocolitis, malrotation with volvulus, and inherited coagulopathy. It is important to note that 15% of necrotizing enterocolitis occurs in full-term babies; malrotation can present simply in shock, without initial overt bleed. Inherited conditions may not be known to the family early on, as they have not yet heard back from the neonatal screening done at birth.
Pitfalls in the neonate and infant
Genitourinary bleeding; hematuria; or uric acid crystals: the classic fake out here is the orange or pink stained diaper – that is actually residue from deposits of uric acid crystals in the urine, an almost always benign phenomenon in which the concentrated crystals oxidize and stain the diaper, frightening the parents.
Think -- pink stain, without clot:

Infants and Young Children
Well appearing?
Through the first year to age 5, things like infectious colitis and gastritis are common.
Ill appearing?
Think about intussusception, cryptic liver disease, or esophageal bleeding. Check the skin – is that a dark purple palpable rash on the buttocks? Think Henoch-Schoenlein purpura.
Focus: Meckel's Diverticulum
Meckel’s diverticulum is the most common congenital malformation of the GI tract, and the most common cause of GI bleeding in the toddler. It is a remnant of the omphalomesenteric tract – it came from a long tube that once connected the yolk sac to the lumen of the midgut. A stranded island of gastric tissue secretes acid in the intestine, where it doesn’t belong. Sometimes these islands never cause much trouble.
When it does present itself, a Meckel’s diverticulum usually follows the rule of twos:
Presents by age 2
Affects 2% of the population
Often 2 inches in length
May include 2 types of mucosa
Found within 2 feet of the ileocecal valve.
Not actively bleeding: technetium-99 pertechnate scintigram (Meckel’s scan).
Actively bleeding: radio-labeled red blood-cell scan (resuscitate and call your surgeons!)
Pitfalls in the infant and young child
Epistaxis; food-related misadventures
Older Child and Adolescent
Well appearing?
Mallory-Weiss tears after forceful vomiting; trivial hemoptysis after viral symptoms; pill esophagitis in the child is just learning to swallow medications. Always consider foreign body ingestion.
Ill Appearing?
Varices from cryptic liver disease; hemorrhagic gastritis; vascular malformation, such as a Dieulafoy lesion, where a tortuous small artery ends just superficial to the gastric mucosa, and can erode through and erupt.
Focus: Inflammatory Bowel Disease
Approximately a quarter of patients with inflammatory bowel disease (IBD) -- both Ulcerative Colitis and Crohn disease – will present by age 20. Children and adolescents may present with the classic symptoms of IBD: abdominal pain, weight loss, bloody diarrhea, but many present atypically with isolated signs like poor growth, anemia, or delayed puberty.
You may also suspect IBD in the child with other extra-intestinal symptoms like oral ulcers, clubbing, erythema nodosum, jaundice, or hepatomegaly.
On history and physical examination, you may get one of three cardinal presentations
Fatigue, history of anemia, in a stable child who comes to the ED with bloody diarrhea
Chronic diarrhea, chronic abdominal pain, and poor weight gain or weight loss
A fulminant presentation, with severe abdominal pain, frankly bloody stools, tenesmus, fever, leukocytosis, and hypoalbuminemia.
On exam, look for general appearance, glossitis from B2 deficiency, hair loss and brittle nails form protein loss, purpura (from vitamin C and vitamin K deficiencies). Look for evidence of episcleritis or uveitis. Listen for rubs as in pericarditis. Do a good abdominal exam, especially looking for hepatomegaly. Perirectal skin tags are not uncommon. Children with IBD may form urinary calculi form oxalate crystal deposition. Do a thorough skin and neurologic exam.
Treatment for both ulcerative colitis and Crohn’s disease is similar.
Induction therapy: children with mild disease get aminosalicylates; those with moderate disease get steroids; and those with severe disease get cyclosporine.
Maintenance regimens to prevent relapse include aminosalicylates, mercaptopurine, and azathioprine.
Surgical treatments for refractory colitis include an ileal pouch and anal anastomosis – also called a J pouch, a type of neorectum created surgically by folding loops of ileum back on themselves and stitching them together to create a larger rectal reservoir where the rectum once was. A neorectum allows the child to have voluntary control of his stools again.
Stabilizing the Pediatric GI Bleed
Life-threatening GI bleeding in children is, thankfully, rare, but we have to be prepared.
Give blood for compensated shock, prepare for massive transfusion if giving more than 40 mL/kg total blood products.
Differing Etiologies: adults and children
The reasons for upper GI bleed in adults are vastly different from those of children. In adults, mostly the life-threats are due to liver disease, varices, or hemorrhagic gastritis.
In children, critical upper GI bleed is often secondary to critical illness (hemorrhagic gastritis or stress ulcer), or vascular malformation. Critical lower gastrointestinal bleeding may be from Meckel's diverticulum or other congenital angiodysplasia.
Endoscopy
Get your patient urgent endoscopy as soon as possible after arrival if there is active bleeding. Otherwise, according to the Belgian guidelines, stable children may have endoscopy within the first 24 hours of hospitalization. The reported efficacy of endoscopy for controlling upper GI bleeding in children is approximately 90%.
Miscellaneous
Nasogastric tube? No routine role (unreliable to rule out or stratify upper GI bleed).
Proton pump inhibitor? No good data, but no major common contraindications.
Octreotide, vasopressin, broad-spectrum antibiotics? May use adult data to extrapolate in the proper etiologic context.
General Advice for the Brisk GI Bleed in Children
This is a rare, but potentially life threatening situation, so anticipate how the child can decline, and get your team assembled: your pediatric intensivist, gastroenterologist, and surgeon – especially if we can’t ge the upper GI bleed to abate with endoscopy. The sooner you activate the team, the better.
Summary
The broad differential diagnosis may be paralyzing, and frustrating, since much of it we cannot discern in the ED.
Consider actionable, high-yield etiologies based on age and appearance.
Neonates
Well appearing? Think rectal fissure, maternal blood, or allergic proctocolitis
Ill appearing? Think necrotizing enterocolitis, malrotation, or an inherited coagulaopathy.
Infants, and Young Children
Well appearing? Think infectious colitis and gastritis.
Ill appearing? Think intussusception and Meckel’s diverticulum.
Older Children and Adolescents
Well appearing? Think Mallory Weiss tear from vomiting, or gastritis
Ill appearing? Think metabolic, cryptic liver disease, or inflammatory bowel disease.
For everyone – a careful history and a good physical exam will point you tto the etiology, or risk-stratify for further outpatient evaluation and management.
References
This post and podcast are dedicated to Carlo D'Apuzzo, MD for his creativity, innovation, and dedication to the highest standards of emergency care. Le tue pillole sono buona medicina. Grazie, Carlo per tutto quello che fai per il mondo #FOAMed.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
Seemingly vague, but potentially dangerous...
common, but possibly with consequences...
...or maybe just plain frustrating.
Let's talk risk stratification, diagnosis, and management.
Primary or Secondary?
We can make headache as easy or as complicated as we like, but let's break it down to what we need to know now, and what the parents need to know when they go home.
Primary headaches: headaches with no sinister secondary cause – like tension or migraine – are of course diagnoses of exclusion (cluster headache is exceedingly rare in children).
Secondary headaches: headaches due to some underlying cause -- are what we need to focus on first.
The list of etiologies is vast; here is just a sampling:
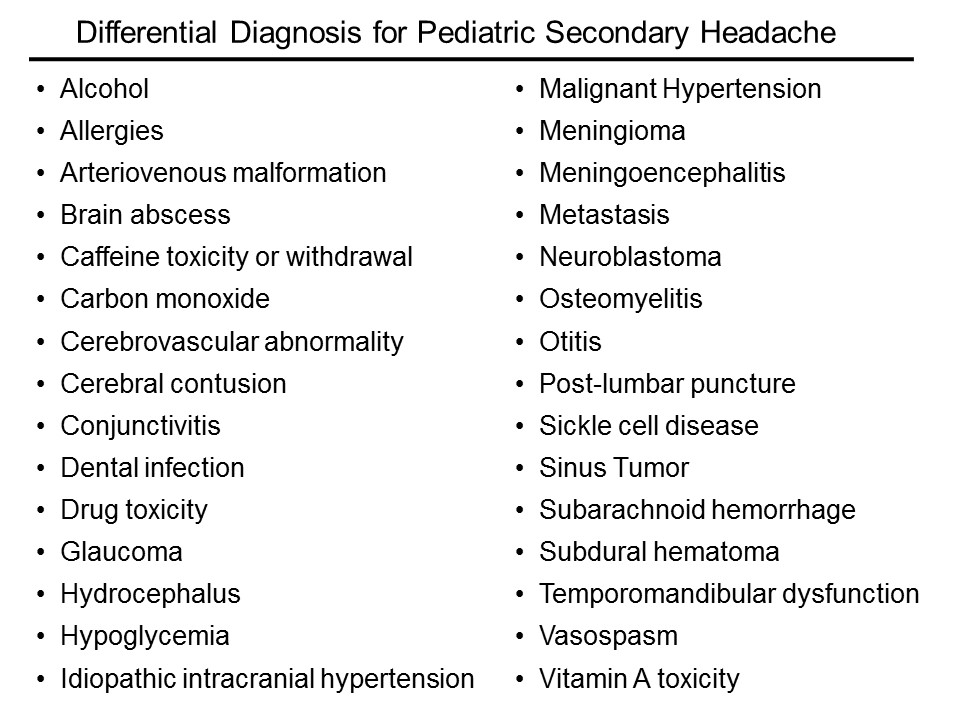
How do I sort this out?
Ask yourself three main questions:
Is it a tumor?
Is it an infection?
Is it a bleed?
Is it a tumor?
Some historical features are high-yield in screening for signs or symptoms consistent with a space occupying lesion.
Progression and worsening of symptoms over time
Associated vomiting
Pain only in the occiput
Headache that is worse with Valsalva – ask if coughing, urinating, or defecating affects the headache
Does this headache wake the child from sleep?
Is it worse in the morning just after getting up?
Conversely, the absence of some historical features may increase suspicion of a space-occupying lesion
No family history of migraine
No associated aura with the headache.
Who needs neuroimaging?
The short answer is, if the child has an abnormal exam finding, then obtain a non-contrast head CT in the ED. If you’re worried enough to get imaging, then you should not feel great about sending him to an expedition to MRI.
The reassuring point is that for a child with a normal neuro exam, we have time to figure this out. For the recurrent headache, outpatient MRI really is the way to go if at all possible – not only do we forgo unnecessary radiation, but MRI is more likely to reveal the cause – or rule out the concern.
Medina et al. in Pediatrics reported on children with headache suspected of having a brain tumor. They stratified patients into low, intermediate, and high risk, based on clinical predictors from the history and physical. All had imaging. They then calculated probability of tumor in each group.
The low risk group had a 0.01% probability of tumor. The intermediate group 0.4%, and the high-risk group had only a 4% probability of tumor. The take-home message is that in the stable patient with a normal neurologic exam and no red flags, time is on our side.
The American Academy of Neurology's most recent guidelines, published first in 1994 and revised in 2004.
1. Neuroimaging on a routine basis is not indicated with recurrent headaches and a normal neurologic exam
2. Neuroimaging should be considered in children with an abnormal exam.
3. Neuroimaging should be considered in children with recent onset of severe headache, change in the type of headache, or associated features that suggest neurologic dysfunction
Is it an infection?
This is nothing new: if you think you need to perform a lumbar puncture, then you’re right. Go after the diagnosis when it meets your threshold for testing.
The difficulty is in the child who just has a headache, plus or minus symptoms that may be viral syndrome.
Dr Curtis et al. in Pediatrics did a systematic review of Clinical Features Suggestive of Meningitis in Children. In the history, only obvious features were helpful in this study: bulging fontanel in the infant or neck stiffness in the older child. Both increased the likelihood of meningitis by 8-fold.
In the physical examination, the only reliable predictors in this study were poor general appearance or a change in behavior.
You will catch those cases, because you would have tuned into meningitis early on -- especially in the unvaccinated.
What about all-comers with fever and headache? The presence of a high fever (so greater than 40 °C) only conferred a positive likelihood ratio of 2.9, only marginally predictive. Reassuring is that for temperatures less than 40 °C, the LR was 1 for meningitis.
In other words, a fever less than 40 °C was just as likely to be present with or without meningitis.
Is it a bleed?
Does this child have some underlying disorder? For example, sickle cell disease, hypertension, rheumatologic disease, or some other endocrine or metabolic disease, such as a mitochondrial disorder?
In chronically ill children, consider cerebral sinus venous thrombosis, vasculitis, ischemia, or hemorrhage.
Arteriovenous malformation (AVM) is the hemorrhage we fear the most.
We really don’t know enough about arteriovenous malformations in the brain to say what is the typical presentation. They may be completely asymptomatic, until they rupture. Even the headache presentation is variable.
Think, headache PLUS.
New headache plus…vomiting.
Headache plus…it’s unilateral and new for the patient.
Headache plus…a new seizure.
Headache plus…focal neuro deficits, that may be transient, due to a vascular steal phenomenon.
Two illustrative cases of arteriovenous malformation:
1. An eleven-year-old girl presents to the ED with new headache, nausea, and vomting in the morning, then had a generalized seizure later that day, and presents with a low GCS. She was intubated, CT confirmed the AVM. She had a right frontal intraparenchymal bleed with midline shift. She underwent clot evacuation and extirpation of the intertwined arteries and veins.
2. A nine-year old girl presented to the ED with headache for two days, constant, then one day of nausea and vomiting. On presentation, she was altered, and had slow-reacting pupils. She also underwent evacuation, and only on histopathology did they find a single, arterialized vein.
Primary Headache: Presumptive Impression
Tension headaches are the most common in children and adults. As in adults, the tension headache is band-like, pressure, tighetening, and often associated with muscle aches in the neck and shoulders.
Find out how often they occur, and whether there is any pattern of worsening symptoms, or if the symptoms seem to be related to sleep hygiene, video games, too much digital screen time. Also, screen for lack of exercise, poor diet, stress, and all of the other good questions you usually ask.
Treat the cause or counsel about lifestyle modification, and offer PO hydration and an NSAID, like ibuprofen or acetaminophen (paracetamol).
Non-pharmacologic techniques like heat packs, rest, stress relief, and a little TLC always help. Be careful not to encourage overreacting to the headache – sometimes we see a pattern of headache, attention, and more headache that can take root. Also look for overuse of medications, which may be the culprit in up to 50% of chronic headaches. Taking NSAIDs 3 or more times per week is associated with medication-induced headache, or cephalalgia medicamentosa.
We often fail to identify migraine headaches in children in the ED, likely for two reasons: prevalence of migraine increases with age, and children don’t present exactly like adults.
Stewart et al. in Neurology, report a prevalence of migraine in children that increases with age: 3 to 7 years of age was 2%; 7 to 11 years of age, 7%; and 11 to 20 years of age, 20%
Pearl: migraines are most commonly bilateral and temporal in children. They resemble "adult" tension headaches, but are much more severe.
We may not be able to sort this out in the ED. The point here is that migraines in children are more common that we may expect, and they can interfere with school performance, with social development, or even with family dynamics and overall stress burden.
Primary Headache Diagnosis: Not (Usually) "Our Thing"
You noticed that we treated before we knew exactly the etiology; such is Emergency Medicine.
We may not be able to make a specific, definitive primary headache diagnosis in the ED, but we should be aware of the criteria to help counsel patients and families.
Tension headache is the most common, but it requires multiple, similar episodes:
Migraine headache (without aura) requires less episodes, but more specific features:
An aura is a fast-pass to diagnosis of migraine:
Primary Headache Management
So how do we treat primary headaches? If you feel this is a mild tension headache, fluids by mouth and a simple NSAID are probably all that is needed, in addition to a heaping dose of reassurance. Ibuprofen (10 mg/kg/dose q 6h, up to 600 mg) for a short course has the most evidence basis. Acetaminophen (paracetamol) (15 mg/kg/dose q6 h) for a short course may also be given.
Abortive treatments with the triptans may have been tried at home, but if they are coming to see us, we are past the point where triptans will be helpful.
For the primary headache that is resistant to NSAIDs, IV therapy may be considered.
If you’re going for IV, a nice evidence-based migraine cocktail is the following:
1. A bolus of 20 ml/kg of normal saline, up to a liter
2. Ketorolac (0.5 mg/kg; max, 30 mg)
3. Diphenhydramine (2 mg/kg; max, 50 mg)
4. Prochlorperazine (0.1 mg/kg; max, 10 mg)
Dr Kaar et al. in Pediatric Emergency Care evaluated the safety and efficacy of their institution’s standardized pediatric migraine practice guideline in the emergency department, which used ths cocktail, based on the best evidence available. In their retrospective chart review, they found the average visual pain scale drop from 7.8 to 2.1
There were no adverse events reported.
So, really you can treat children with migraines very similarly to adults.
Other treatments on the horizon (still under investigation) in children include IV adjuncts such as magnesium, valproic acid, and dexamethasone.
Aftercare and Recurrence Prevention
For everyone who is going home, take just a moment to talk about the importance of sleeping well, eating well, getting exercise, limiting digital screen time, and trying to improve ways of dealing with stress.
When all else fails, and the parent has “heard it all”: get them started on a headache diary.
Take a piece of paper, fold it in half, and start a template for them to work on in a spiral notebook. Start a sample entry for them, with the date and time the headache started, what it felt like, what was happening just before, what made the headache better, any dose of medications given, how long it lasted, and what the patient did after. There are even free apps that will track the headache pattern.
This is the first thing a neurologist will start them on – and it’s sometimes a selling point to the parent that the time spent waiting for a referral to a neurologist is not waste – they will actually be in better shape and can move things along faster. It also gives them some sens of control of what can be a draining situation.
Summary and Mental Road Map
If you were thinking meningitis or acute bleed, especially with fever or meningismus, get a CT first if you see signs of increased intracranial pressure, or if there is an abnormal neuro exam. Otherwise go straight to the lumbar puncture (LP).
In the afebrile child with a normal exam, give symptomatic relief, briefly counsel them, and arrange for follow-up.
In the afebrile child with an abnormal exam, obtain a CT in the ED. If negative, either admit for MRI if you are still concerned, or consider LP for idiopathic intracranial hypertension (pseudotumor cerebri).
Talk with parents early about expectations, and offer them some friendly advice on prevention. Refer patients to the primary care provider or neurologist if the presentation is more involved.
After a good history and physical examination in the ED that results in no red flags, we have time on our side. Help the family through the process by explaining the next steps and what can be done in the meantime. Compassion and a plan: sometimes these are our most powerful allies.
References
This post and podcast are dedicated to Mark Wilson, PhD, BSc, MBBChir, FRCS(SN), MRCA, FIMC, FRGS for his #FOAMed generosity, candor, humility, and dedication to the care of the acutely ill and injured. Thank you.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
Have you ever been in any of these situations?
⇒ You have a stable child who just needs fluids, but no laboratory tests
⇒ You’ve tried PO hydration, to no avail, despite anti-emetics
⇒ You’re poking the stable, but dehydrated child repeatedly without success
What now?
Hypodermoclysis, otherwise known as subcutaneous rehydration.
[Insert Player]
Clysis comes from the same Greek word that “a flood” – hypodermoclysis refers to flooding the subcutaneous space with fluid, so that it can be absorbed systemically.
Sound far-fetched?
Well, it turns out, what is old is new again.
In 1913, Dr Day first described this technique for a child with severe diarrhea who could not tolerate fluids by mouth. Hypodermoclysis then began to gain popularity with a peak of use in the 1940s, until an innovative breakthrough in 1950. Dr David Massa, a resident anesthesiologist at the Mayo clinic, invented the first catheter-over-needle apparatus.
With increasing safety and ready access of IV catheters, IV quickly overshadowed SC.
The subcutaneous route of hydration has also been used effectively in geriatric and palliative care for decades, and it is only now beginning to gain popularity again in its original population: children.
So, how does it work?
In a nutshell, you place a butterfly needle or angiocatheter in the subcutaneous space and you run fluids into it. The tissues quickly absorb the fluids, making them available systemically. That’s it. Everything else is just finesse.
The ideal candidate for hypodermoclysis is the stable patient, with mild to moderate dehydration who fails a trial of fluids by mouth, or who needs a bridge to gaining IV access later, after a slow subcutaneous fluid bolus is given.
Ok, so how do you do it?
Place a topical anesthetic cream, such as EMLA, cover with occlusive dressing (IV dressing), wait 15-20 min
"Pinch an inch" of skin anywhere, but the most practical site in young children is between the scapulae
Insert a 25-gauge butterfly needle or 24-gauge angiocatheter (preferred by the author), secure
Inject 150 U hyaluronidase SC, if available
Infuse 20 mL/kg isotonic solution over one hour, repeat as needed or use "bolus" as bridge to IV access
You can set the line to gravity, and if it is dripping in, you may leave it be. If you see a very slow drip by gravity, or worse, nothing is dripping, you can set the line on a pump, to deliver up to 20 mL/kg over an hour. Infusion at this rate optimizes the balance we want in minimal discomfort while maximizing the flow rate.
This is not a “bolus” in the true sense – but then, when you compare it to the alternative – like IV therapy – and we see a time and cost savings. Dr Mace and colleagues in the American Journal of Emergency Medicine report substantially decreased cost and ED length of stay when comparing the material and human resources needed to place an IV in a squirmy young child, compared with a simple subcutaneous stick.
There will be swelling
There will be swelling – that is the goal. It is really painless, and your patient may lie down on his back with the pump going – it is actually pretty comfortable for most children and adults to do.
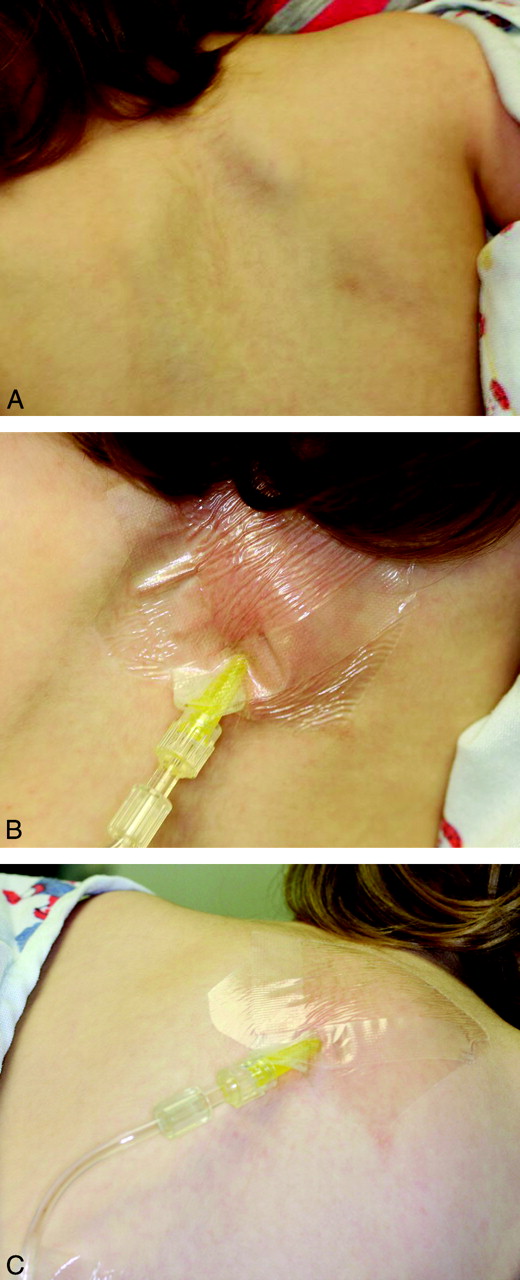 Here’s a tip – since there will be swelling, we want to be careful about how we secure the line, so how you tape it down to the skin is important – we want to avoid a pulling sensation, which can be the beginning of the end of the tolerance for the procedure. Cover that with an occlusive dressing, as you would an IV site. The footprint of the occlusive dressing is relatively small, so it will travel up on top of the subcutaneous mound you’re creating. As the line exits the occlusive patch, place a thin layer of gauze between the skin and the IV tubing, so that the tubing doesn’t press into the skin. Then—as far away from the puncture site as possible—tape it down securely. The idea is not to tape on the growing mound itself, because the mound may pull at the anchored skin and set a nuclear chain reaction of annoyance and restlessness – and potentially a failed procedure.
Here’s a tip – since there will be swelling, we want to be careful about how we secure the line, so how you tape it down to the skin is important – we want to avoid a pulling sensation, which can be the beginning of the end of the tolerance for the procedure. Cover that with an occlusive dressing, as you would an IV site. The footprint of the occlusive dressing is relatively small, so it will travel up on top of the subcutaneous mound you’re creating. As the line exits the occlusive patch, place a thin layer of gauze between the skin and the IV tubing, so that the tubing doesn’t press into the skin. Then—as far away from the puncture site as possible—tape it down securely. The idea is not to tape on the growing mound itself, because the mound may pull at the anchored skin and set a nuclear chain reaction of annoyance and restlessness – and potentially a failed procedure.
The swelling will look indurated, a pinkish red. It’s not an allergic reaction: even with the old preparations of hyaluronidase, allergic reactions were rare, and now they are very rare with the recombinant preparation. It is supposed to swell and look ugly. The subcutaneous tissues will swell to a point where you have a steady state fluid administration rate, and as soon as you stop the infusion, the remaining fluid will start to subside as it is absorbed.
A Bridge to IV Therapy?
Kuensting et al. in the Journal of Emergency Nursing in 2013 compared subcutaneous fluid infusion with intravenous fluid infusion in children with difficult IV access. They found the mean time from order entry to subcutaneous fluid infusion to be 20 min, compared to the failed IV access group with an average infusion start time of 1.5 hours. The latter group eventually received subcutaneous fluids. The investigators also found a shorter ED length of stay in the subcutaneous group.
In the same study, a subgroup received subcutaneous fluids initially, and later an IV. They found a trend in ease of IV access after subcutaneous fluid therapy. In other words, if your little patient with difficult IV access is hemodynamically stable and amenable to a bolus over an hour, you may choose to start with hypodermoclysis and reevaluate.
Predicting Difficult IV Access in Children
Much has been studied and written about the predictors of difficult IV access in children. The most often cited are: age < 3 years, weight less than 5 kg, prematurity, obesity, and darker skin tones, where the contrast of vein to skin may not be so apparent.
The three main predictors of the score validated by Riker et al. in Annals of Emergency Medicine include the most practical and universal of features: vein palpability, vein visibility, and patient age.
If you’re anticipating difficult IV access in the child who can stand to wait an hour for a slow bolus, you may start with the subcutaneous route to get those veins plumper and more visible, to improve your chances of IV access in the very near future.
Medications via Subcutaneous Route
Certain medications have been used safely via subcutaneous infusion; always check dose, rate, and compatibility.
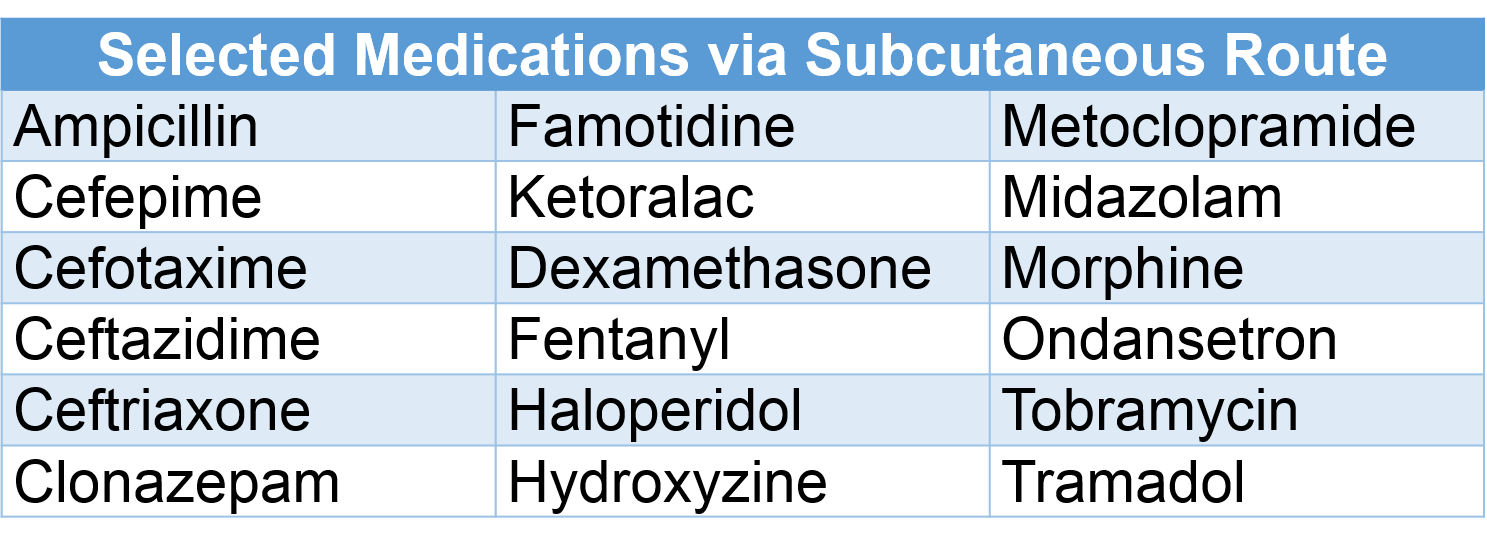
What about catheter size?
You don’t need to use larger needles or angiocathters for older children, adolescents or adults. A 25-gauge butterfly or 24-gauge angiocatheter works well from an infant to an elder. In one study of adults, a half a liter of saline was infused by gravity via a 24-gauge catheter. With IVs, the shorter and larger the bore, the faster the infusion.
In subcutaneous infusion, it is not the size of the catheter, but the osmotic gradient that determines the rate of absorption.
What if I don't have that fancy hyaluronidase?
It’s actually increasingly readily found – and available in generic form. If you have it, please use it – it will make a believer out of you and others.
Hypodermoclysis will work without hyaluronidase – the process of subcutaneous rehydration just takes a lot longer to work. In a double-blind cross-over trial Thomas et al. in 2007 compared subcutaneous administration of lactated ringer’s solution by gravity with and without hyalurondase. The hyaluronidase group received their fluids 5 times faster. The average rate of the hyaluronidase group was 382 mL/h versus the fluid only group, who did not receive hyalurinodase; they were substantially slower, at 82 mL/h. It’s worth using if you have it, but still potentially useful if you don’t.
Recap: Supplies
√ EMLA or any topical anesthetic used for intact skin, placed as soon as the decision is made
√ A 25-gauge butterfly needle or 24-gauge angiocatheter
√ IV tubing, gauze to pad, tape to anchor
√ 150 U hyaluronidase, the same dose, regardless of age or size
√ Isotonic fluids – you can start with 20 ml/kg
√ And finally a well informed team made up by the patient, the parents, and your staff, so that everyone knows what to expect for a successful subcutaneous fluid administration.
References
Smith LS. Hypodermoclysis with older adults. Nursing. 2014 Dec;44(12):66.
This post and podcast are dedicated to Christina L. Shenvi, MD, PhD, for her dedication to excellence in patient care and enthusiasm in #FOAMed, Emergency Medicine, and Geriatric Emergency Medicine. There are many shared lessons learned in the care of children, elders, and families. Thank you.
Catch Dr Shenvi on the innovative GEMcast.
Subcutaneous Infusion
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
"She won't walk", or "He just looks like he's limping".
So many things can be going on -- how do we tackle this chief complaint?
You’re dreading a big work-up. You almost want to tell the kid – please, STOP LIMPING...
STOP LIMPING!
S – Septic Arthritis
The most urgent part of our differential diagnosis. The hip is the most common joint affected, followed by the knee. Lab work can be helpful, as well as US of the hip to look for an effusion, but sometimes, regardless of the results, the joint just has to be tapped to know for sure.
T – Toddler’s fracture
This is usually a torque injury when the wobbling toddler pivots quickly or trips and falls. Toddler’s fractures happen in children 1 to 3 years of age, and occur in the distal 1/3 of the tibia. Sometimes a cast is needed, but currently there is a new trend in foregoing casting in mild cases.
O – Osteomyelitis
Bacteremia – from any source – can seed into any bone. It’s not very common, but it happens: approximately 2% of children who present to an ED with limp will have osteomyelitis. Plain films, ESR, and CRP are a fair screen to start. For more than the casual concern, MRI is the best modality to evaluate, followed by radionuclide scintigraphy. Although not the first choice modality, CT can show periosteal changes, such as inflammatory new bone formation or periosteal purulence.
P – Perthes disease
This is the famous Legg-Calvé-Perthes idiopathic avascular necrosis of the hip, usually affecting children from 3 to 12 years. They present with a slow onset pain and with an antalgic gait. Patients will have trouble with internal rotation and abduction of the hip. Radiographs may be initially normal. MRI can show the culprit: decreased perfusion to the femoral head and subsequent necrosis.
L – Limb-Length Discrepancy
Parents may notice that he seems “wobblier” than he should be. It may be that we are just now appreciating a congenital anomaly. Get out the paper tape, and measure from the anterior superior iliac spine to the medial malleolus and compare both sides. Children with limb-length discrepancy only need a non-urgent referral to pediatric orthopedics to look for congenital dysplasia of the hip, or other growth abnormalities. Some are treated with orthotics. Surgical options vary. Epiphysiodesis destroys the growth plate on the unaffected side, which evens out the growth. Other options are limb-lengthening or limb-shortening procedures.
I – Inflammatory
Transient Synovitis. This is what we want them to have right? The typical age is between 3 and 6 years, sometimes just after a URI. To be comfortable with this diagnosis, we should have considered all of the dangerous diagnoses, the child should be well, afebrile, in minimal discomfort, and he should respond almost completely to an NSAID. He’s the one running up and down the department after treatment – or just from sheer boredom after observation.
M – Malignancy
Primary bone tumors such as Ewing’s sarcoma or osteogenic sarcoma typically affect older children. Limping, however, may be a presenting symptom of leukemia. If you have any suspicion of the general wellness of the child, get a screening CBC, and perhaps a peripheral blood smear. Whatever you do, make sure you get close follow up for these kids that are on your malignancy radar -- the blast crisis may not have occurred yet – but it can happen hours to days later.
Plain films are insensitive for leukemic involvement of bone but they may show diffuse osteopenia, or metaphyseal bands – symmetrical high-uptake markings around the joint. They look like stacks of paper within normal bone – you can see them also in anemia, lead poisoning, and other causes. Also look for periosteal new bone formation, sclerosis, or lysis.
P – Pyomyositis
This usually presents with vague irritability, pain, and fever, and sometimes with a subacute minor trauma. These children don’t look to well.
Also think about just run-of-the-mill myositis, usually from a viral cause, such as influenza. Typically the calves are affected and are always tender. Hydration and supportive therapy are indicated for viral causes.
For bacterial focal pyomyositis, give empiric antibiotics, admit them for major inpatient workup, and think about early surgical consultation if you think you need sepsis source control.
I – Iliopsoas Abscess
Children most often will develop a primary abscess from bacteremia from an unresolved infection. Adults more commonly form secondary abscesses from Crohn’s disease, post-op complications, a vertebral infection, or even a bad chronic urinary tract infection. Lest you think this is a dramatic presentation, think again: iliopsoas abscesses present also with vague symptoms of back, flank, abdominal, or hip pain, sometimes with fever. The median time from symptoms to diagnosis in children is a whopping 20+ days, according to one study. If iliopsoas abscess is starting to get your attention, get the CT or MRI.
N – Neurologic
Not to scare you, but children do have strokes; unlike adults, half are hemorrhagic, half are thromboembolic. Typically they’ll have some underlying pathology that will alert you, such as a cardiac lesion, sickle cell disease, or some infectious or metabolic history. The good news is that it won’t just be a limp – you’ll have some other neuro sign or symptom to go after.
Guillain Barré is another thing to consider – early lower extremity weakness may present as a limp or refusal to walk. Maybe it’s not the hip that should be tapped, but the spinal canal.
Think also about muscular dystrophy or peripheral neuropathy and its possible underlying etiology.
G – Gastrointestinal and Genitourinary
What else could be going on? Appendicitis may be faking you out here. Perhaps there is a hernia, or testicular or ovarian torsion, all of which can present as lateralizing pain and not wanting to walk. Think outside the box.
Phases of Gait
The gait cycle has three phases: contact, stance, and propulsion. Contact is the time from heel strike to just when the foot is flat. Stance is from the foot being flat to lifting the heel from the ground. The stance phase is when you bear most of your weight. The propulsion phase is when your weight transfers to your toes, and you push off.
Abnormal Gaits
Antalgic Gait -- "hobbling" gait; normal contact phase, but stance phase is abbreviated; propulsion is normal. The patient is trying to limit the time spent bearing weight on that side.
Trendelenburg Gait -- the affected side's hip abductor muscles are too weak or painful to stabilize the pelvis; the unaffected side dips to the floor. May be superior gluteal neuropathy, or a biomechanical problem, such as avascular necrosis, congenital dysplasia of the hip, or slipped capital femoral epiphysis.
Circumduction Gait -- the patient swings his foot laterally (due to a foot or ankle pathology), or to avoid tripping in limb-length discepancy.
Stiff-leggged Gait -- the patient walks with knees locked, in an attempt to avoid using the gastrocnemius muscles; concerning for myositis.
Equinus Gait -- toe-walking, as seen in myositis, also to avoid exacerbating pain from the calves.
 Lag of Internal Rotation of the Hip
Lag of Internal Rotation of the Hip
Look for symmetry of internal rotation, or lateralizing pain or "guarding" with range of motion.
Keep the pelvis flush to the bed, and simultaneously rotate the lower extremity laterally, which will cause internal rotation of the hip.
Avascular necrosis will not allow full internal rotation, since the joint space is narrowed with this maneuver, causing impingement of the sensitive necrotic head of the femur.
Note any pain, asymmetry, and angle of internal rotation achieved.
Kocher Criteria
In their original paper in 1999, Dr Kocher et al. performed a retrospective analysis of children who were being evaluated for a septic joint versus transient synovitis over a 15 year period, in a major referral center. They came up with four independent predictors of a septic joint, and calculated the probability of septic arthritis based on the number of features present. In 2004 the same group validated their prediction tool, with a slightly decreased sensitivity and specificity in the validation population.
In short, the Kocher criteria are not perfect, but it’s the best evidence we have at the moment.
The four predictors are:
Inability to walk
Fever of 38.5 C of greater
ESR > 40 mm/h
WBC > 12,000
Bonus mnemonic: Walk FEW: Inability to Walk | Fever | ESR | WBC
The probability of septic arthritis increases with increasing predictor. In this prediction model, each predictor has the same weight.
Probability of Septic Arthritis (Kocher et al. 1999)
0 Predictor – <0.2 %
1 Predictor – 3%
2 Predictors – 40%
3 Predictors – 93.1%
4 Predictors – 99.6%
Now, remember, this is to be used in children in whom you already have some suspicion of a septic joint. So, 0 predictors, generally you’re alright. 1 predictor, you may start to worry. Once you have 2 predictors, your chances jump for 3% to 40%. You really have to go looking.
The Kocher caveat is that there is no single test or single decision rule that will stop you from investigating if you are concerned enough. Don’t have too much faith in this imperfect decision tool – we use it because we need somewhere to start. Treat and push for the aspiration of the hip if you are left in doubt. Septic arthritis can be devastating if not identified early.
Summary
- Fever and limp? – do not pass go – especially with 2 or more Kocher criteria – or if there is any doubt, tap that joint.
- Refusal to walk after adequate analgesia? Admit for observation, MRI, further workup, blood cultures, and much more.
- Remember some developmental concerns to help you to decide whether to continue the investigation urgently as an inpatient, or non-urgently as an outpatient.
- After all of that, try to get them them to STOP LIMPING!
References
Lindsay D, D'Souza S. A limping child. BMJ. 2016 Feb 9;352:i476.
Verma S. Pyomyositis in Children. Curr Infect Dis Rep. 2016 Mar;18(4):12.
This post and podcast are dedicated to the estimable yet graciously humble Andrew Tagg BSC(Hons), MBBS, MRCSEd, ACEM for his dedication to #FOAMed, Emergency Medicine, Pediatric Emergency Medicine, and all things caffeinated. Thank you for your dedication, generosity, and your example.
Don't Forget the Conference! #DFTB17 #DFTB
Do we recognize shock early enough?
How do we prioritize our interventions?
How can we tell whether we’re making our patient better or worse?
World wide, shock is a leading cause of morbidity and mortality in children, mostly for failure to recognize or to treat adequately.
So, what is shock?
Simply put, shock is the inadequate delivery of oxygen to your tissues. That’s it. Our main focus is on improving our patient’s perfusion.
Oxygen delivery to the tissues depends on cardiac output, hemoglobin concentration, the oxygen saturation of the hemoglobin you have, and the environmental partial pressure of oxygen.
At the bedside, we can measure some of these things, directly or indirectly. But did you notice that blood pressure is not part of the equation? The reason for that is that blood pressure is really an indirect proxy for perfusion – it’s not necessary the ultimate goal.
The equation here is a formality:
DO2 = (cardiac output) x [(hemoglobin concentration) x SaO2 x 1.39] + (PaO2 x 0.003)
Shock CAN be associated with a low blood pressure, but shock is not DEFINED by a low blood pressure.
Compensated Shock: tachycardia with poor perfusion. A child compensates for low cardiac output with tachycardia and a increase in systemic vascular resistance.
Decompensated Shock: frank hypotension, an ominous, pre-arrest phenomenon.
Shock is multifactorial, but we need to identify a primary cause to prioritize interventions.
How they "COHDe": Cardiogenic, Obstructive, Hypovolemic, and Distributive.
Cardiogenic Shock
All will present with tachycardia out of proportion to exam, and sometimes with unexplained belly pain, usually due to hepatic congestion. The typical scenario in myocarditis is a precipitous decline after what seemed like a run-of-the-mill URI.
Cardiogenic shock in children can be from congenital heart disease or from acquired etiologies, such as myocarditis. Children, like adults, present in cardiogenic shock in any four of the following combinations: warm, cold, wet, or dry.
"Warm and Dry"
A child with heart failure is “warm and dry” when he has heart failure signs (weight gain, mild hepatomegaly), but has enough forward flow that he has not developed pulmonary venous congestion. A warm and dry presentation is typically early in the course, and presents with tachycardia only.
"Warm and Wet"
If he worsens, he becomes “warm and wet” with pulmonary congestion – you’ll hear crackles and see some respiratory distress. Infants with a “warm and wet” cardiac presentation sometimes show sacral edema – it is their dependent region, equivalent to peripheral edema as we see in adults with right-sided failure.
“Warm” patients – both warm and dry and warm and wet -- typically have had a slower onset of their symptoms, and time to compensate partially. Cool patients are much sicker.
"Cold and Dry”
A patient with poor cardiac output; he is doing everything he can to compensate with increased peripheral vascular resistance, which will only worsen forward flow. Children who have a “cold and dry” cardiac presentation may have oliguria, and are often very ill appearing, with altered mental status.
"Cold and Wet"
The sickest of the group, this patient is so clamped down peripherally that it is now hindering forward flow, causing acute congestion, and pulmonary venous back-up. You will see cool, mottled extremities.
Cardiogenic Shock: Act
Use point-of-care cardiac ultrasound:
Good Squeeze? M-mode to measure fractional shortening of the myocardium or anterior mitral leaflet excursion.
Pericardial Effusion? Get ready to aspirate.
Ventricle Size? Collapsed, Dilated,
Careful with fluids -- patients in cardiogenic shock may need small aliquots, but go quickly to a pressor to support perfusion
Pressor of choice: epinephrine, continuous IV infusion: 0.1 to 1 mcg/kg/minute. Usual adult starting range will end up being 1 to 10 mcg/min.
Avoid norepinephrine, as it increases systemic vascular resistance, may affect afterload
Just say no to dopamine: increased mortality when compared to epinephrine
Obstructive Shock
Mostly one of two entities: pulmonary embolism or cardiac tamponade.
Pulmonary embolism in children is uncommon – when children have PE, there is almost always a reason for it – it just does not happen in normal, healthy children without risk factors.
Children with PE will either have a major thrombophilic comorbidity, or they are generously sized teenage girls on estrogen therapy.
Tamponade -- can be infectious, rheumotologic, oncologic, or traumatic. It’s seen easily enough on point of care ultrasound. If there is non-traumatic tamponade physiology, get that spinal needle and get to aspirating.
Obstructive Shock: Act
Pulmonary embolism (PE) with overt shock: thrombolyse; otherwise controversial. PE with symptoms: heparin.
Tamponade: if any sign of shock, pericardiocentesis, preferentially ultrasound-guided.
Hypovolemic Shock
The most common presentation of pediatric shock; look for decreased activity, decreased urine output, absence of tears, dry mucous membranes, sunken fontanelle. May be due to obvious GI losses or simply poor intake.
Rapid reversal of hypovolemic shock: may need multiple sequential boluses of isotonic solutions. Use 10 mL/kg in neonates and young infants, and 20 mL/kg thereafter.
Hypovolemic Shock: Act
Tip: in infants, use pre-filled sterile flushes to push fluids quickly. In older children, use a 3-way stop cock in line with your fluids and a 30 mL syringe to "pull" fluids, turn the stop cock, and "push them into the patient.
Titrate to signs of perfusion, such as an improvement in mental status, heart rate, capillary refill, and urine output.
When concerned about balancing between osmolality, acid-base status, and volume status, volume always wins. Our kidneys are smarter than we are, but they need to be perfused first.
Distributive Shock
The most common cause of distributive shock is sepsis, followed by anaphylactic, toxicologic, adrenal, and neurogenic causes. Septic shock is multifactorial, with hypovolemic, cardiogenic, and distributive components.
Children with sepsis come in two varieties: warm shock and cold shock.
Distributive Shock: Act
Warm shock is due to peripheral vascular dilation, and is best treated with norepinephrine.
Cold shock is due to a child’s extreme vasoconstriction in an attempt to compensate. Cold shock is the most common presentation in pediatric septic shock, and is treated with epinephrine.
Early antibiotics are crucial, and culture everything that seems appropriate.
Shock: A Practical Approach
"How FAST you FILL the PUMP and SQUEEZE"
Sometimes things are not so cut-and-dried. We'll use a practical approach to diagnose and intervene simultaneously.
Look at 4 key players in shock: heart rate, volume status, contractility, and systemic vascular resistance.
How FAST you FILL the PUMP and SQUEEZE
First, we look at heart rate -- how FAST?
Look at the heart rate – is it sinus? Could this be a supraventricular tachycardia that does not allow for enough diastolic filling, leading to poor cardiac output? If so, use 1 J/kg to synchronize cardiovert. Conversely, is the heart rate too slow – even if the stroke volume is sufficient, if there is severe bradycardia, then cardiac output -- which is in liters/min – is decreased. Chemically pace with atropine, 0.01 mg/kg up to 0.5 mg, or use transcutaneous pacing.
If the heart rate is what is causing the shock, address that first.
Next, we look at volume status.
How FAST you FILL the PUMP and SQUEEZE
Look to FILL the tank if necessary. Does the patient appear volume depleted? Try a standard bolus – if this improves his status, you are on the right track.
Now, we look at contractility.
How FAST you FILL the PUMP and SQUEEZE
Is there a problem with the PUMP? That is, with contractility? Is this in an infarction, an infection, a poisoning? Look for signs of cardiac congestion on physical exam. Put the probe on the patient’s chest, and look for effusion. Look to see if there is mild, moderate, or severe decrease in cardiac contractility. If this is cardiogenic shock – a problem with the pump itself -- begin pressors.
And finally, we look to the peripheral vascular resistance.
How FAST you FILL the PUMP and SQUEEZE
Is there a problem with systemic vascular resistance – the SQUEEZE?
Look for signs of changes in temperature – is the patient flushed? Is this an infectious etiology? Are there neurogenic or anaphylactic concerns? After assessing the heart rate, optimizing volume status, evaluating contractility, is the cause of the shock peripheral vasodilation? If so, treat the cause – perhaps this is a distributive problem due to anaphylaxis. Treat with epinephrine. The diagnosis of exclusion in trauma is neurogenic shock. Perhaps this is warm shock, both are supported with norepinephrine. All of these affect systemic vascular resistance – and the shock won’t be reversed until you optimize the peripheral squeeze.
Summary
The four take-home points in the approach to shock in children
- To prioritize your innterventions, remember how patients COHDe: Cardiogenic, Obstructive, Hypovolemic, and Distributive. Your patient's shock may be multifactorial, but mentally prioritize what you think is the MAIN case of the shock, and deal with that first.
- To treat shock, remember: How FAST You FILL The PUMP and SQUEEZE: Look at the heart rate – how FAST. Look at the volume status – the FILL. Assess cardiac contractility – the PUMP, and evaluate the peripheral vascular tone – the SQUEEZE.
- In pediatric sepsis, the most common type is cold shock – use epinephrine (adrenaline) to get that heart to increase the cardiac output. In adolescents and adults, they more often present in warm shock, use norepinephrine (noradrenaline) for its peripheral squeeze to counteract this distributive type of shock.
- Rapid-fire word association:
- Epinephrine for cardiogenic shock
- Intervention for obstructive shock
- Fluids for hypovolemic shock
- Norepinephrine for distributive shock
References
This post and podcast are dedicated to Natalie May, MBChB, MPHe, MCEM, FCEM for her collaborative spirit, expertise, and her super-charged support of #FOAMed. You make a difference. Thank you.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
How do you approach the child who may be altered?
Altered mental status in children can be subtle. Look for age-specific behaviors that range from irritability to anger to sleepiness to decreased interaction.
In the altered child, anchoring bias is your biggest enemy. Keep your mind open to the possibilities, and be ready to change it, when new information becomes available.
For altered adults, use AEIOU TIPS (Alcohol-Epilepsy-Insulin-Overdose-Uremia-Trauma-Infection-Psychosis-Stroke).
Try this for altered children: remember that they need their VITAMINS!
V – Vascular (e.g. arteriovenous malformation, systemic vasculitis)
I – Infection (e.g. meningoencephalitis, overwhelming alternate source of sepsis)
T – Toxins (e.g. environmental, medications, contaminated breast milk)
A – Accident/abuse (e.g. non-accidental trauma, sequelae of previous trauma)
M – Metabolic (e.g. hypoglycemia, DKA, thyroid disorders)
I – Intussusception (e.g. the somnolent variant of intussusception, with lethargy)
N – Neoplasm (e.g. sludge phenomenon, secondary sepsis, hypoglycemia from supply-demand mismatch)
S – Seizure (e.g. seizure and its variable presentation, especially subclinical status epilepticus)
Case One: Sleepy Toddler
16-month-old who chewed on his grandmother's clonidine patch
Clonidine is an alpha-2 agonist with many therapeutic indications including hypertension, alcohol withdrawal, smoking cessation, perimenopausal symptoms. In children specifically, clonidine is prescribed for attention deficit hyperactivity disorder, spasticity due to cerebral palsy and other neurologic disorders, and Tourette’s syndrome.
The classic clonidine toxidrome is altered mental status, miosis, hypotension, bradycardia, and bradypnea. Clonidine is on the infamous list of “one pill can kill”.
Treatment is primarily supportive, with careful serial examinations of the airway, and strict hemodynamic monitoring.
Naloxone can partially counteract the endogenous opioids that are released with clonidine's pharmacodynamics.
Start with the usual naloxone dose of 0.01 mg/kg, up to the typical adult starting dose is 0.4 mg.
In clonidine overdose, however, you may need to increase the naloxone dose (incomplete and variable activity) up to 0.1 mg/kg. Titrate to hemodynamic stability and spontaneous respirations, not full reversal of all CNS effects.
Case Two: In Bed All Day
A 7-year-old with fever, vomiting, body aches, sick contacts. Altered on exam.
Should you get a CT before LP?
If you were going to perform CT regardless, then do it.
Adult guidelines: age over 60, immunocompromised state, history of central nervous system disease, seizure within one week before presentation, abnormal level of consciousness, an inability to answer two consecutive questions correctly or to follow two consecutive commands, gaze palsy, abnormal visual fields, facial palsy, arm drift, leg drift, and abnormal language.
Children: if altered, and your differential diagnosis is broad (especially if you may suspect tumor, bleed, obvious abscess).
Influenza is often overlooked as a potential cause of altered mental status. Many authors report a broad array of neurological manifestations associated with influenza, such as altered mental status, seizures, cranial nerve abnormalities, hallucinations, abnormal behavior, and persistent irritability. All of this is due to a hypercytokinemic state, not a primary CNS infection.
Case Three: Terrible Teenager
14-year-old brought in for "not listening" and "acting crazy"; non-complaint on medications for systemic lupus erythematosus (SLE).
SLE is rare in children under 5. When school-age children present with SLE, they typically have more systemic signs and symptoms. Teenagers present like adults. All young people have a larger disease burden with lupus, since they have many more years to develop complications.
Lupus cerebritis: high-dose corticosteroids, and possibly IV immunoglobulin. Many will need therapeutic plasma exchange (TPE), a type of plasmapheresis.
Summary
- In altered mental status, keep your differential diagnosis open
- Pursue multiple possibilities until you are able to discard them
- Be ready to change your mind completely with new information
- Make sure your altered child gets his VITAMINS (Vascular, Infectious, Toxins, Accident/Abuse, Metabolic, Intussusception, Neoplasm, Stroke)
References
Zorc JJ. A lethargic infant: Ingestion or deception? Pediatr Ann 2000; 29: 104–107
This post and podcast are dedicated to Teresa Chan, HBSc, BEd, MD, MS, FRCPC for her boundless passion for and support of #FOAMed, for her innovation in education, and for her dedication to making you and me better clinicians and educators. Thank you, T-Chan.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
A 5-year-old boy was playing with his older brother in front of their home when he was struck by a car. He sustained a femur fracture, splenic laceration, and blunt head trauma – the so-called Waddell’s triad.
On arrival, he was in compensated shock, with tachycardia.
He decompensates and needs blood.
How do we manage his hemodynamics and when do we perform massive transfusion?
Pediatric Massive Transfusion
40 mL/kg of blood products given at any time within the first 24 hours.
Adolescents and Adult Massive Transfusion
6-8 units of packed red blood cells (PRBCs)
- Adults have about 5 L of circulating blood.
- Not including plasma, one could replace all circulating erythrocytes with about 10 units of PRBCS
- The best ratio of PRBCs:Plasma:Platelets is unknown, but consensus is 1:1:1.
- 1 unit of PRBCS is typically 300 mL of volume.
The typical initial transfusion of PRBCs in children is 10 mL/kg.
Massive transfusion in children is defined as 40 mL/kg of any blood product.
Once you start to give a child with major trauma the second 10 mL/kg dose of PRBCs – start thinking about other blood components, and ask yourself whether you should initiate your massive transfusion protocol.
The goal is to have the products ready to use in the case of the dynamic trauma patient.
The Thromboelastogram (TEG)
Direct measures the four components of clot formation. When there is endolethial damage and bleeding, the sequence that your body takes to address it is as follows:
- Platelets migrate and form a plug
- Clotting factors aggregate and reinforce the platelets
- Fibrin arrives an acts like glue
- Other cells migrate and support the clot.
R time – reaction time – the initial line in the tracing that shows time to beginning of clot formation.
- Treated with platelets
K factor – kinetics of the clot –how much the clot allows the pin to move, or the amplitude.
- Treated with cryoprecipitate
Alpha angle – the slope between the R and K measurements – reflects how quickly the fibrin glue is working.
- Treated with cryoprecipitate
Ma – maximum amplitude – reflects the overall strength of the clot.
- Treated with platelets
LY30 – the clot lysis at 30 min – is the decrease in strength of the clot’s amplitude at 30 min.
- Treated with an antifibrinolytics (tranexamic acid)
Shape Recognition
Red wine glass: a normal tracing with a normal reaction time and a normal amplitude. That patient just needs support and monitoring.
Champagne glass: a coagulopathic TEG tracing – thinned out, with less amplitude. This patient needs specific blood products.
Puffer fish or blob: a hyperfibrinolytic tracing. That patient will needs clot-stablizer.
TEG – like the FAST – can be repeated as the clinical picture changes.
The Trauma Death Spiral
Lethal triad of hypothermia, acidosis, and coagulopathy.
Keep the patient perfused and warm.
Each unit of PRBCs contains 3 g citrate, which binds ionized calcium, causing hypotension. In massive transfusion, give 20 mg/kg of calcium chloride, up to 2 g, over 15 minutes. Calcium chloride is preferred, as it is ionically readily available – just use a larger-bore IV and watch for infiltration. Calcium gluconate could be used, but it requires metabolism into a bioavailable source of calcium.
Prothrombin complex concentrate (PCC)
Prothrombin complex concentrate (PCC) is derived from pooled human plasma and contains 25-30 times the concentration of clotting factors as FFP. Four-factor PCCs contain factors II, VII, IX and X, while 3-factor PCCs contain little or no factor VII.
The typical dose of PCC is 20-50 units/kg
In the severely hemorrhaging patient – you don’t have time to wait for the other blood products to thaw – PCC is a powder that is reconstituted instantly at the bedside.
Tranexamic acid (TXA)
Tranexamic acid (TXA), is an anti-fibrinolytic agent that functions by stopping the activation of plasminogen to plasmin, and the degradation of fibrin. The Clinical Randomisation of an Antifibrinolytic in Significant Hemorrhage (CRASH-2) investigators revealed a significant decrease in death secondary to bleeding when TXA was administered early following trauma.
Based on the adult literature, one guideline is to give 15 mg/kg loading dose of TXA with a max 1 g over 10 minutes followed by 2 mg/kg/h for at least 8 h or until bleeding stops.
Resuscitative Pearls
Our goal here is damage control. Apply pressure whenever possible. Otherwise, resuscitate, identify the bleeding source, and slow or stop the bleeding with blood products or surgery.
How Children are Different in Trauma
In adults, we speak of “permissive hypotension” (also called “balanced resuscitation” or “damage control resuscitation”). The idea is that if we bring the adult patient’s blood pressure up to normal, we may be promoting clot rupture. To avoid this, we target a MAP of 65 and look for clinical signs of sufficient perfusion. Adults tolerate hypotension relatively well, and is sufficient until we send them to the OR or interventional radiology suite.
In children, this is simply not the case. Hypotension in children is a sign of pre-arrest. Remember, they compensate with an increased systemic vascular resistance and tachycardia to maintain blood pressure.
We should not allow children to become hypotensive – severe tachycardia alone should prompt us to resuscitate.
In other words, permissive hypotension is not permissible for children.
FAST is not sensitive enough to rule-out abdominal trauma.
Fox et al in Academic Emergency Medicine found a sensitivity of 52%; with a 95% confidence interval [CI] = 31% to 73%.
Often children even with high-grade splenic and liver lacerations can be managed non-operatively. If they are supported adequately, they are observed in the ICU and can avoid surgery in many cases. Unfortunately, a negative FAST cannot help with detecting or grading the laceration for non-operative management. In other words, feel free to use ultrasound – especially for things that we in the ED will react to and intervene on – but CT may help to manage the traumatized child non-operatively.
General Guideline for Imaging in Pediatric Trauma
CT Head and Neck, non-contrast: in concerning mechanisms of injury, patients that are difficult to assess (especially those under 3 months), those with a GCS of 13 or lower.
CT Chest, IV contrast: for suspicion of vascular injury that needs exploration, especially in penetrating trauma. Otherwise, chest xray will tell you everything you need to know in children – especially in blunt trauma. Hemo or pneumothoraces are readily picked up by US or CXR. Rib fractures on CXR predict pulmonary contusions. If you are concerned about great vessel injury, then CT Chest may be helpful; otherwise consider omitting it.
CT Abdomen and Pelvis, IV contrast: helpful in grading splenic and liver lacerations with goal to manage non-operatively. Abdominal tenderness to palpation, significant bruising, or a seat belt sign are concerning and would generally warrant a CT. Also, consider in liver function test abnormalities, or hematuria.
Extremity injuries: in general can be evaluated with physical exam and plain films. However, some injuries in high-risk anatomically complex areas such as the hand and wrist, tibial plateau, and midfoot may be missed by plain films, and CT may be helpful here.
Remember: you can help to mitigate post-traumatic stress and risk for adult healthcare aversion.
Summary
- Massive transfusion in children is at 40 mL/kg of total blood products. Think about it if you are giving your second transfusion to the traumatized child.
- Do everything you can to support perfusion and avoid the death spiral of hypothermia, coagulopathy, and acidosis. Keep the child perfused with blood as needed, correct coagulopathy, avoid too much crystalloid, and make sure to use the least high-tech of all of these interventions – keep him dry and covered with warm blankets.
- Do a careful physical exam, and use CT selectively with an end-point in mind – the default is not the pan-scan – evaluate possible injuries depending on your suspicions from history, physical, and lab tests.
- Become familiar with the relatively new modalities in trauma such as TXA, cryoprecipitate and the emerging technology of thromboelestogram – red wine is good for you, champagne is weak, and a puffer fish is trouble.
Selected References
This post and podcast are dedicated to Larry Mellick, MS, MD, FAAP, FACEP. Thank you for your dedication to medical education, and sharing your warm bedside manner, extensive knowledge and talents, and your patient interactions with the world.
Powered by #FOAMed — Tim Horeczko, MD, MSCR, FACEP, FAAP
Traumatized children need your full attention.
Protocols work well for adults, but trauma in children requires that we exercise our clinical muscles just a bit more.
Two main reasons:
-
Children have specific injury patterns
-
Their physiologic response to trauma is unique.
Crash course in pediatric anatomy and physiology in trauma
When you think of trauma in children, think of Charlie Brown. Large head, no neck, his chest and abdomen form an underdeveloped, amorphous shape.
Alternatively, think of children as apples – they are rounder than they are tall, with a large increased surface area. Apples don’t have a hard shell or thick rind to protect them. If you drop them, you may not see any evidence of damage to the outside, but there can be considerable bruising just under the surface.
- A child has thin skin, less subcutaneous deposits than an adult, and a non-calcified, pliable thorax that deforms more than it protects or shields.
- The child’s abdominal muscles are not yet developed. There is less peritoneal fat to cushion a blow, and so traumatic forces transmit readily into internal organs, often without external bruising.
- The child’s large surface area also causes him to dissipate heat more quickly. He may be wet from urine or blood, and in a major trauma, this faster cool-down predisposes him to coagulopathy.
Case
A 5-year-old boy who was playing with his older brother in front of their home when the ball rolled into the street. He ran after it, and was struck by a sedan going approximately 30 mph.
This is the so-called Wadell’s triad that occurs in a collision of auto versus pedestrian or auto versus bicycle. The initial impact is the greatest, and will vary depending on the child’s height and what part of his body reaches up to the bumper of the car. Depending on the height of the child and the height of the car, the initial impact will cause a femur fracture, a pelvic fracture, or direct abdominal trauma. The second impact happens as the child is flung onto the grill or the hood of the car, causing usually thoracic trauma. The third impact can be the coup de grace – to add insult to major injury, the child is then propelled forward, worsening the two previous impacts’ injuries and adding a third – severe blunt head trauma.
Intubation Pearl #1:
If your patient has any subtle change in mental status, intubate early. In pediatric trauma, we need to be proactive. Hypoxia is our enemy.
Intubation Pearl #2:
Thankfully cervical spine injuries in children are uncommon, and when they do occur, they typically occur at the child’s fulcrum, which is at C2. Compare this with an adult’s injury pattern with our fulcrum at C7. Be careful and minimize manipulation of the cervical spine, but do what you must to visualize the chords and place the tube. Keep the neck midline, and realize that the child’s usual decrease respiratory reserve is even more affected by trauma. Preoxygenate and pass that tube quickly.
Chest Tube Pearl #1:
Chest tube sizing in pediatrics is straightforward if we remember that the traditional chest tube size is 4 x the ETT size.
Chest Tube Pearl #2:
Try using a pigtail catheter.
Safety Triangle
- Lateral edge of the pectoral muscle
- Lateral edge of the latisimus dorsi
- Line along the fifth intercostal space at the level of the nipple.
It’s roughly where you would put on a generous dose of deodorant. Insertion here minimizes the risk of damage to nerves, vessels and organs.
Resuscitative Thoracotomy in Children
In a 40-year review of ED thoracotomy, Moore et al. analyzed 1,691 patients who received ED thoracotomy. Overall all-cause adult survival was 6.1%. In children ? 15 years of age, overall all-cause survival was considerably less, at 3.4%.
In a large case series and review of the literature for pediatric ED thoracotomy, Allen et al. found a survival rate in penetrating trauma of 10.2%, with a much lower survival rate in blunt pediatric arrest, at 1.6%. Adolescents had more penetrating injuries, and younger children had more blunt trauma.
To synthesize, the rarity of ED thoracotomy in children is due to the fact that:
- Traumatic full arrest in children is uncommon.
- It is most often blunt trauma.
- Blunt traumatic arrest in children is mostly non-survivable.
REBOA
If you have access to resuscitative endovascular balloon occlusion of the aorta or REBOA, this may be an option to temporize the child to get him to the relative control of the operating room. REBOA involves accessing the common femoral artery, passing a vascular sheath, floating a balloon catheter to the appropriate section of the aorta, and inflating the balloon to occlude blood flow.
Brenner et al. described a case series of 6 patients from two Level I trauma centers. They used REBOA for refractory hemorrhagic shock due to either blunt or penetrating injury. After balloon occlusion, blood pressure improved sufficiently to take the patient either to interventional radiology or to the OR. Four patients lived, two died. The AORTA trial is underway to investigate its use in trauma.
Summary:
- Children are like Charlie Brown – large head, no neck, amorphous, underdeveloped and unprotected thorax and abdomen. Or, if you like, they’re like, apples – they have a large surface area and are easily internally bruised, often without overt signs of external bruising.
- Chest tubes for children are very similar to the adult procedure – the traditional chest tube size is 4 x the child’s ETT size. Try to use smaller pigtail catheters, available in commercial kits, whenever possible. They’re easy, safe, and effective.
- Resuscitative thoracotomy is for penetrating trauma with signs of life wthin 10-15 minutes of arrival. Find the correctable surgical cause of the arrest. Resuscitative thoracotomy for blunt trauma has a dismal prognosis in children.
Selected References
Pediatric Trauma on WikEM
This post and podcast are dedicated to Dr Al Sacchetti, MD, FACEP. Thank you for promoting the emergency care of children and for spreading the message that you don’t need subspecialty training to take good care of acutely ill and injured children.
Powered by #FOAMed — Tim Horeczko, MD, MSCR, FACEP, FAAP
Victims of electrical injuries present either in extremis or as the seeming well patient with insidious, developing disease.
A targeted history usually gets you the information you need.
Four main things to find out:
1. Household or Industrial electricity?
Household electricity uses alternating current, or AC. Voltages across the world range anywhere from 100 to 240 V. Here in North America, most outlets and appliances use 120 volts, which is the measure of electrical tension, or the potential difference in electrical charge.
Cut-off between low voltage and high voltage is 1000 V.
Industrial energy may be AC or direct current, DC. DC current propels the victim -- think of this as a blast injury. The same voltage in AC is three times as damaging as that voltage at DC, because AC causes muscle tetany, and prolonged contact time.
2. What was the likely pathway that current took?
Did the current pass through the thorax? -- Think dysrhythmias. Through the head or neck? -- Think damage to the CNS and risk for later central respiratory arrest; acoustic nerve damage; cataract formation. Did the current pass along an extremity? -- Think compartment syndrome and rhabdomyolysis.
3. What was the contact time?
The electrical charge meets resistance and converts to thermal energy, which causes tissue necrosis, increasing with the contact time. Was your patient extricated? Was there tetany? Was he found in a pool of water or liquid? Longer contact time correlates with extensive injuries that may only be apparent hours later.
4. Are there any associated injuries?
Think of electrical injury as a trauma – major trauma rarely occurs in isolation. Was the patient flung after contact? Did he have a syncopal episode? -- Think precipitated dysrhythmia and fall. Was there any chest pain? -- Consider stress-induced ischemia.
Pearl: Patients may be confused initially or unable to localize symptoms because of CNS disruption. Get collateral information, re-interview, and re-examine as needed.
Case 1: Toddler with an oral commissure burn
An electrical burn to the angle of the mouth cauterizes superficial bleeding vessels, and hours later the wound becomes covered with a white layer of fibrin, surrounded by erythema. Edema and thrombosis will continue, and at 24 hours there is typically a significant margin of tissue necrosis. Most patients do well, and the burn heals by secondary intention. The eschar will slough off in 1 to 2 weeks. The labial artery is just deep to the burn, and as the eschar sloughs off, it can be exposed. It’s a high-flow artery to the face, and if disrupted, the child may have significant bleeding and possibly hemorrhagic shock.
These children need close wound care follow-up, and potentially outpatient coordination with Head and Neck Surgery and/or Plastic Surgery consultants.
Precautionary advice: take the moment to talk to parents about the risk, and show them how to apply pressure to the wound, pinching the inner and outer cheek together with the thumb and index finger until the child arrives to the hospital.
Case 2: School-age child with knife versus electrical outlet
A a “kissing burn” occurs when the electrical charge creates an arc and jumps to a more proximal portion of the extremity.
The kissing burn typically occurs at flexor creases such as the wrist or the antecubital fossa. There is often extensive underlying tissue damage even under the skin where it doesn’t appear to be involved. Compartment syndrome and subsequent rhabdomyolysis and renal failure are the highest-risk complications.
Case 3: Adolescent after a taser exposure
Nitrogen capsules propel two barbs into the dermis, which deliver short bursts of energy; most patients have no harm from the electricity delivered.
How to remove a dart: The darts are typically 9 mm long, but the small barb is typically not buried very deep in the skin. Hold the skin taught, use a hemostat to grasp the end as close to the skin as possible, align the dart perpendicularly to the skin, and pull quickly and firmly.
If the patient can’t tolerate this or the barb appears particularly embedded, inject with local lidocaine and make a small superficial incision with an 11-blade scalpel just large enough to allow passage. Ultrasound can be used to troubleshoot when needed.
Taser dispo: People who have been tased do remarkably well and complications are rare. In a review of tasers used by law enforcement, Vilke et al. found that there was no need for routine laboratory testing or observation, as there was ‘no evidence of dangerous laboratory abnormalities, physiologic changes, or immediate or delayed cardiac ischemia or dysrhythmias after exposure to electrical discharges of up to 15 s.” Subsequent studies with minors less than 16 years of age found similar results.
Special note on the patient with agitated delirium or stimulant intoxication: treat these patients carefully, as the organic problem that got them tased in the first place still needs to be addressed, and substances such as PCP, cocaine, and methamphetamines are all cardiac irritants and may predispose them to dysrythmias.
Case 4: Adolescent in full arrest after lightening strike
Patients who are struck by direct current like lightening should be treated aggressively, because the reason for their cardiac arrest is often reversible if treated quickly. Either the current sent the victim into a dysrhythmia, or it caused a temporary paralysis of the thoracic muscles, resulting in a primary respiratory arrest.
For victims of a lightning strike, classically we use reverse triage – normally, those in full arrest are triaged as black, deceases. In high-voltage and lightening injuries, we tend to those in full arrest first, because you might quickly reverse them, and can move on to the next patient triaged red, or immediate.
High-voltage injuries are a multi-trauma – other sequelae include pulmonary edema, paralysis, ileus, and cataracts, in addition to the more immediate cardiac, musculoskeletal, neurologic, and renal considerations.
Regardless of the exposure, obtain an ECG and look for bundle branch block, heart block, and dysrhythmias, since those will change disposition. In those who are injured, consider a basic metabolic panel, looking for potassium, calcium, and creatinine. A creatine phosphokinase or total CK will screen for rhabdomyolysis. Troponin is not predictive of the extent of direct myocardial damage, but get it if you think there might be a stress-induced, or type II MI. Radiography as needed depending on the presenting associated trauma.
Take Home Points
1. Injury from electrical burns can be subtle. Think of patients as having occult multi-trauma. Be thorough in history and examination. Plan to re-examine either during observation in the ED, or in close outpatient follow-up.
2. Discharge patients with low-voltage injury, no symptoms, and a normal ECG. Counsel outpatients and provide close follow-up as appropriate.
3. Admit patients with low-voltage injury with signs or symptoms such as loss of consciousness, ECG changes, or evidence of end-organ damage on laboratory screening. Admit all patients with high-voltage injury, even if asymptomatic and a normal laboratory screen.
4. Transfer patients with high-voltage injury or significant burns to a regional burn center or trauma center.
References
Celik A, Ergün O, Ozok G. Pediatric electrical injuries: a review of 38 consecutive patients. J Pediatr Surg. 2004;39(8):1233-1237.
Ericsson KA. Deliberate Practice and Acquisition of Expert Performance: A General Overview. Acad Emerg Med. 2008; 15:988-994.
Fish RM. Electric injury, part I: treatment priorities, subtle diagnostic factors, and burns. J Emerg Med. 1999;17(6):977-983. doi:10.1016/S0736-4679(99)00127-4.
Fish RM. Electric injury, part II: Specific injuries. J Emerg Med. 2000;18(1):27-34. doi:10.1016/S0736-4679(99)00158-4.
Fish RM. Electric injury, part III: cardiac monitoring indications, the pregnant patient, and lightning. J Emerg Med. 2000;18(2):181-187. doi:10.1016/S0736-4679(99)00190-0.
Horeczko T. “Electrical Injuries: Shocking or Subtle?” In Avoiding Common Errors in the Emergency Department, 2nd Edition. Mattu M, Swadron SP (eds). Lippincott, Williams & Wilkins. Phiadelphia. 2016. (In Press).
Rai J, Jeschke MG, Barrow RE, Herndon DN. Electrical Injuries: A 30-Year Review. J Trauma Acute Care Surg. 1999;46(5):933-936.
Vilke GM, Bozeman WP, Chan TC. Emergency department evaluation after conducted energy weapon use: review of the literature for the clinician. J Emerg Med. 2011; 40(5):598-604.
This post and podcast are dedicated to Joelle Donofrio, MD, FAAP for her tireless care of children, in the ED and in the field. A special thank you and dedication to Cliff Reid, BM, FRCP(Glasg), FRCSEd(A&E), FRCEM, FACEM, FFICM, FCCP, EDIC, DCH, DipIMC, RCSEd, DipRTM, RCSEd, CCPU, CFEU for his transformative intelligence and educational verve.
Pediatric airway management is a skill that integrates the three types of knowledge as described by the ancient Greeks: episteme, or theoretical knowledge, techne, or technical knowledge, and phronesis, or practical wisdom, also called prudence.
Here we’ll invoke each type of knowledge and understanding as we go beyond the anatomical issues in pediatric airway management – to the advanced decision-making aspect of RSI and the what-to-do-when the rubber-hits-the road.
Case 1: Sepsis
Laura is a 2-month-old baby girl born at 32 weeks gestational age who today has been “breathing fast” per mother. On arrival she is in severe respiratory distress with nasal flaring and intercostal retractions. Her heart rate is 160, RR 50, oxygen saturation is 88% on RA. She has fine tissue-paper like rales throughout her lung fields. Despite a trial of a bronchodilator, supplemental oxygen, even nasal CPAP and fluids, she becomes less responsive and her heart rate begins to drop relatively in the 80s to 90s – this is not a sign of improvement, but of impending cardiovascular collapse.
She is in respiratory failure from bronchiolitis and likely viral sepsis. She needs her airway taken over.
Is this child stable enough for intubation?
We have a few minutes to optimize, to resuscitate before we intubate.
Here’s an easy tip: use the sterile flushes in your IV cart and push in 20, 40, or 60 mL/kg NS. Just keep track of the number of syringes you use – it is the fastest way to get a meaningful bolus in a small child.
Alternatively, if you put a 3-way stop-cock in the IV line and attach a 30 mL syringe, you can turn the stop cock, draw up stream from the IV bag into the syringe, turn te stop cock, and push the fluid in the IV.
Induction Agent in Sepsis
The consensus recommendation for the induction agent of choice for sepsis in children is ketamine.
Etomidate is perfectly acceptable, but ketamine is actually a superior drug to etomidate in the rapid sequence intubation of children in septic shock. It rapidly provides sedation and analgesia, and supports hemodynamic stability by blocking the reuptake of catecholamines.
Paralytic Agent in Sepsis
The succinylcholine versus rocuronium debate…
Succinylcholine and its PROS
- 82% of RSI in the ED used succinylcholine (According to the National Emergency Airway Registry, in 2005). We know it, we are comfortable with it.
- Succinylcholine produces superior intubating conditions when comparing 1 mg/kg succinylcholine versus 0.6 mg/kg rocuronium, succinylcholine is that at 45 seconds.
Succinylcholine and its CONs
- Raises serum potassium in everyone, typically 0.5 to 1 mEq/L. That is not usually a problem, but for those with preexisting or inducible hyperkalemia, it can precipitate an arrest, as in renal failure, underlying neurologic or myopathic conditions like multiple sclerosis, muscular dystrophy, ALS, or those who had a stroke or a burn more than 72 hours prior. We often have limited information in critical situations.
- Succinylcholine gives us a false sense of security. In children, there really is no “safe apnea” period.
- Succinylcholine’s effect on the nicotinic receptors results in mydriasis, tachycardia, weakness, twitching and hypertension, and fasciculations (Think nicotine overdose: M/T/W/Th/F).
- Succinylcholine’s effect on muscarinic receptors manifest (as in organophosphate overdose): SLUDGE – salivation, lacrimation, urination, defecation, GI upset or more apropos here: DUMBBELLS – diarrhea, urination, miosis, bradycardia, emesis, lacrimation, lethargy, salivation.
- Second dose of succinylcholine – beware of the muscarinic effects and bradycardia. Co-administer atropine, 0.01 mg/kg, up to 0.5 mg IV.
Coda: succinylcholine is not that bad – we would not have had such great success with it during the early years of our specialty if it were such a terrible drug. The side effects are rare, but they can be deadly. So, what’s the alternative?
Rocuronium and its PROs
- It has none of the side-effects of succinylcholine
Rocuronium and its CONs
- Argument 1: the duration is too long if there is a difficult airway, since rocuronium can last over an hour.
Still need to intubate, and now your patient is potentially worse. - Argument 2: succinylcholine produces better intubating conditions at 45 seconds compared to rocuronium.
At 0.6 mg/kg, rocuronium is inferior to succinylcholine at all time intervals. At 1.0 mg/kg, rocuronium is still inferior at 45 seconds. At 1.2 mg/kg rocuronium – the dose now commonly recommended – there was no difference in intubating conditions, per a study by Heier et al. in Anethesia and Analgesia in 2000.
Case 2: Multitrauma
Joseph is a 3-year-old boy who is excited that there are so many guests at his house for a family party and when it’s starting to wind down and the guests begin to leave, he is unaccounted for. An unsuspecting driver of a mini-van backs over him.
He is brought in by paramedics, who are now bagging him.
Induction Agent in Trauma
- Need something that is hemodynamically stable – agents such as midazolam or propofol would cause too many problems.
- Etomidate is a short-acting imidazole derivative that acts on GABA-A receptors to induce loss of consciousness in 5-15 seconds. It can cause apnea, pain on injection, and myoclonus.
- Etomidate reduces cerebral blood flow, reduces intracranial pressure, and reduces cerebral oxygen consumption, all while maintaining arterial blood pressure and cerebral perfusion pressure.
- Ketamine is reasonable as well: there is no contraindication to ketamine except for known hydrocephalus. It is safe in head trauma. It is a good choice for the hypotensive trauma patient. TBI is not a contraindication.
- In the case of the critically injured child who is normotensive, ketamine will raise his blood pressure and perhaps foster further bleeding. The goal is a good general perfusion and a balanced resuscitation, ensuring enough cerebral perfusion without disrupting nascent clots. On the other side of the spectrum, permissive hypotension is not described in children, as hypotension is a late and dangerous sign of shock.
Paralytic Agent in Trauma
Are your surgeons in an uproar about a long-acting agent and the pupillary response? Relax, it’s a myth.
Caro et al in Annals in 2011 reported that the majority of patients undergoing RSI preserved their pupillary response. Succinylcholine actually performed worse than rocuronium. In the rocuronium group, all patients preserved their pupillary response.
In the critically ill, we rethink your dosing of both the sedative and the paralytic.
In a critically ill child or adult, perfusion suffers and it affects how we administer medications. The patient’s arm-brain time or vein-to-brain time is less efficient; additionally, as the patient’s hemodynamic status softens, he becomes very sensitive to the effects of sedatives.
We need to adjust our dosing for a critically ill patient:
- Decrease the sedative to avoid falling over the hemodynamic compensation cliff.
- Increase the paralytic to account for prolonged arm-brain time.
Case 3: Cardiac/myocarditis/congenital heart disease
Jacob is a 6-year-old-boy with tricuspid atresia s/p Fontan procedure who’s had one week of runny nose, cough, and now 2 days of high fever, vomiting, and difficulty breathing.
The Fontan procedure is the last in a series of three palliative procedures in a child with complex cyanotic congenital heart disease with a single-ventricle physiology.
The procedure reroutes venous blood to flow passively into the pulmonary arteries, because the right ventricle has been surgically repurposed to be the systemic pump. The other most common defect with an indication for a Fontan is hypoplastic left heart syndrome.
Typical “normal” saturations are 75 and 85% on RA. Ask the parents or caregiver.
Complications of the Fontan procedure include heart failure, superior vena cava syndrome, and hypercoagulable state, and others.
A patient with a Fontan can present in cardiogenic shock from heart failure, distributive shock from an increased risk of infection, hypovolemic shock from over-diuresis or insensible fluid loss – or just a functional hypovolemia from the fact that his venous return is all passive – and finally obstructive shock due to a pulmonary thromboembolism.
Types of shock mnemonic: this is how people COHDe – Cardiogenic, Obstructive, Hypovolemic, Distributive.
Do we give fluids?
Children after palliative surgery for cyanotic heart disease are volume-dependent. Even if there is a component of cardiogenic shock, they need volume to drive their circuit. Give a test dose of 10 mL/kg NS.
Pressors in Pediatric Shock
- Children compensate their shock state early by increasing their SVR.
- Epinephrine (adrenaline) is great at increasing the cardiac output (with minimal increase in systemic vascular resistance; tachycardia) In children the cardiac deleterious effects are not pronounced as in adults. Later when the child is stabilized, other medication such as milrinone (ionotrope and venodilator) can be used.
- Epinephrine is also fantastic for cold shock when the patient is clamped down with cold extremities – the most common presentation in pediatric septic shock.
- Norepinephrine (noradrenaline) is best used when you need to augment systemic vascular resistance, such as in warm shock, where the patient has loss of peripheral vascular tone.
Induction Agent in Cardiogenic Shock
A blue baby – with a R –> L shunt – needs some pinking up with ketamine
A pink baby – with a L –> R shunt – is already doing ok – don’t rock the boat – give a neutral agent like etomidate.
Myocarditis or other acquired causes of cardiogenic shock – etomidate.
Case 4: Status Epilepticus
Jessica is a 10-year-old girl with Lennox-Gastaut syndrome who arrives to your ED in status epilepticus. She had been reasonably controlled on valproic acid, clonazepam, and a ketogenic diet, but yesterday she went to a birthday party, got into some cake, and has had stomach aches – she’s been refusing to take her medications today.
On arrival, she is hypoventilating, with HR 130s, BP 140/70, SPO2 92% on face mask. She now becomes apneic.
Induction Agent in Status Epilepticus
Many choices, but we can use the properties of a given agent to our advantage. She is normo-to-hypertensive and tachycardic. She has been vomiting. A nice choice here would be propofol.
- Propofol as both a sedative and anti-epileptic agent works primarily on GABA-A and endocannabinoid receptors to provide a brief, but deep hypnotic sedation. Side effects can include hypotension, which is often transient and resolves without treatment. Apnea is the most common side-effect.
- Ketamine would be another good choice here, for its anti-epileptic activity.
Paralytic Agent in Status Epilepticus
Rocuronium (in general), as there are concerns of a neurologic comorbidity.
Housekeeping in RSI
What size catheter doe I use? If you know your ETT size, then it is just a matter of multiplication by 2, 3, 4, or 5.
Remember this: 2, 3, 4 – Tube, Tape, Tap
- The NG/OG/Foley is 2 x the ETT – tube
- The ETT should be taped at a depth of 3 x the ETT size – tape
- A chest tube size 4 x the ETT – tap
In summary, in these cases of sepsis, multitrauma, cardiogenic shock, and status epilepticus:
- Resuscitate before you intubate
- Use the agent’s specific properties and talents to your benefit
- Adjust the dose in critically ill patients: decrease the sedative, increase the paralytic
- Have post-intubation care ready: sedation, verification, NG/OG/foley
EMS is bringing you a child with a VP shunt, port-a-cath, trached on a vent, seizing, hypotensive, and now desaturating – ETA – 3 minutes. Are you ready?
Medicine is evolving. As technology advances, we need to meet the challenge of taking care of our patients who have come to rely on this technology for their basic needs. Before we go further, remember to assess the parent and the child as a unit. The caregiver who is usually the parent, is a rich source of knowledge about the child’s particular condition and past experience. Take them seriously, and be on the lookout for caregiver burnout.
Tracheostomy Troubles
4-month-old baby boy born full term with Pierre Robin Sequence, febrile, not eating anything, now with breathing difficulty.
Place on trach collar oxygen, suction those secretions; flush with small amounts of saline, and repeat.
Any child symptomatic with a trach? Remember to monitor for hypoxia and bradycardia.
Tracheostomy indications: obstruction, primary respiratory compromise, or a neurologic disorder. The obstruction may be a tumor, post-infectious, or addressing a congenital anomaly. Children may have bronchopulmonary dysplasia, a restrictive lung disease such as scoliosis. A wide array of neurologic problems can result in a child’s having a trach, such as cerebral palsy, TBI, or spinal muscular atrophy.
Early complications of trachs especially in the first few months – include bleeding, pneumomediastinum, accidental decanulation, wound breakdown, and subcutaneous emphysema. The most common later complications include infection and granuloma formation. Tracheo-esophageal fistulas and trachea-innominate fistulas are thankfully very rare.
VP Shunt Shudderings
11-year-old girl with a history of prematurity, intraventricular hemorrhage, and subsequent flaccid paralysis with neurogenic bladder. She is brought in by her mother because of constipation and “not acting her usual self”. She is afebrile, abdomen is soft, full of stool.
The most common shunt is the ventriculoperitoneal shunt, originating in a lateral ventricle and tracking subcutaneously down the neck and chest until the distal end enters and coils in the peritoneal space. Less common types include ventriculoatrial, ventriculopleural, ventriculocisternal, ventriculo-vesicular (to gall bladder) and the lumbo-peritoneal, usually reserved for spina bifida.
The common denominator: hydrocephalus. The most common causes are tumor, congenital anomalies, hemorrhage, or post-infectious obstructions.
The two most common complications of VP shunts are malfunction (due to obstruction, fracture, or kinking) or infection. The slit-ventricle syndrome results from overdrainage, causing headaches and ataxia and the slit-ventricle syndrome. An abdominal pseudocyst forms when cells floating in the peritoneal cavity aggregate on the distal tip of the VP shunt, forming a biofilm that fills with CSF. VP shunts, like any foreign body, can migrate and erode through intestines and skin.
Classically in severe hydrocephalus an infant or toddler will have sun-setting eyes – the irises look like a setting sun against the prominent bulbar conjunctiva. However, the presentation is usually much more subtle; if the child just feels off or if the parent tells you he is not acting right, this is a shunt malfunction until proven otherwise.
Garton et al. in the Journal of Neurosurgery followed 344 children with shunts, and found that in the first six months after a shunt is placed, the presence of nausea or vomiting carried a positive LR pf 10.4 for shunt malfunction. Irritability conveyed a positive LR of 9.8 for shunt malfunction. Decreased LOC was 100% predictive.
Most shunt infections occur within a few weeks after placement. 90% of infections occur within the first 9 months. Fever is only 60% sensitive, but CRP is 95% specific.
If the child has severe mental status changes, hypertension, and/or bradycardia, tap the shunt emergently.
Head of the bed is 30 degrees; sterile fashion: don a cap, mask, faceshield, and sterile gloves, chlorhexidine or betadine to clean. Use a 23 or 25 g butterfly attached to a manometer, and advance slowly. Pressures above 25 mmH20 are reliably indicative of a distal shunt obstruction. If there is no return of CSF, or there is poor flow, there probably is a proximal obstruction. Make note of the pressure you get, allow the pressure to equilibrate, remove the needle, and dress it sterilely. Be ready to take over the airway if needed, and use standard ICP lowering temporizing tactics until a neurosurgeon is found.
Vascular Device Dilemmas
A 3-year-old boy with ALL undergoing consolidation chemotherapy has had vomiting with abdominal pain since yesterday; he is febrile, tachycardic, and pale; there is mild tenderness to palpation in the right lower quadrant, and his capillary refill is 3 seconds. He is in compensated shock.
The Huber needle is not a resuscitative line. Obtain proper access to give fluids -- do not rely on the port-a-cath.
Vascular devices are notoriously troublesome. In the European Respiratory Journal, Munck et al. reviewed cases of patients who needed to have their vascular devices out. 43% of them got them out for was for occlusion, 21% infection; other reasons included displacement, rupture, and skin necrosis. Only 2.5% of them were removed for clinical improvement.
When a child or an adult is at risk for a massive air embolism, we should do three things: clamp the device proximal to the fracture or defect, hyperoxygenate, and perform Durant’s maneuver, or left lateral decubitus in Trendelenberg. This forces a presumed air embolus to stay in the apex of the right ventricle until we figure out what to do. You can put the US probe on and look for a whirlwind of tiny bubbles to confirm – it is very sensitive and can detect as little as 0.05 ml/kg of air. Some references advocate for hyperbarics to allow the embolus to resolve, others comment on using a needle to aspirate air. The main thing for us is to suspect it, detect it, control it, and if the child arrests, to do vigorous CPR to mechanically disrupt the bubbles.
G-tube Tumult
A 17-year-old boy with a history of botulism had a rough ICU course, home after rehabilitation, with some residual dysmotility issues, still partially g-tube dependent. No complaints in the ED, but there is irritation around the stoma, and discomfort with g-tube manipulation.
G-tubes are placed for one of three reasons: insufficient intake, increased demand, or increased loss. Insufficient intake may be due to anatomical problems, prematurity, or failure to thrive. Increased demand may be temporary, such as in burns, s/p cardiac surgery, or ay prolonged recovery. Increased losses may be from enteropathies, or short gut syndrome.
Alphabet Tube
Gastrostomy or g-tubes end directly in the stomach. Whatever you can take by mouth can go into the g-tube, including medications and bolus feeds.
Jejunostomy tubes, or J-tubes are placed by IR or surgery for babies with severe reflux – this is for drip feeds, usually done at night.
Gastro-jejunostomy tubes or G-J tubes use the G port for medications, and the J port for continuous feeds.
We do not pull or replace or touch G-J or J tubes in the ED, but we can place a Foley catheter in the stoma to keep it patent if needed.
“Buried bumper syndrome” occurs when the patient has not changed his g-tube for much longer than recommended, or if there is a dramatic change in habitus. The inner balloon is pulled up and away from the stomach lumen, so that it is displaced and fixed – causing pain, inadequate feeds, obstruction, and sometimes peritonitis.
Take Home Messages
Tracheostomy: the stoma matures in one month – you can change out after that, suction, suction, suction, and you can place an ETT if the patient is critical.
Ventriculoperitoneal Shunts: 90% of infections occur within the first 9 months.
Vascular Devices: assume the line is not functional, and use another to resuscitate, especially in port-a-caths.
Gastrostomy Tubes: buried bmpers are bad business – be aware of the painful, obstructed, poorly mobile g-tube. The stoma matures in one month is open, three months of it’s a PEG. If it has fallen out, you have 1-3 hours before the stoma begins to close – temporize with a foley catheter.
When it comes to the technologically dependent child in the ED, familiarity breeds…confidence!
Selected References
Babu R, Spicer RD. Implanted vascular devices (ports) in children: compications and their prevention. Pediatr Surg Int (2002) 18: 50-53
DiBaise JK, Scolapio JS. Home Parenteral and Enteral Nutrition. Gastroenterol Clin N Am 36 (2007) 123-144l
Feinberg A et al. Gastrointestinal Care of Children and Adolescents with Developmental Disabilities. Pediatr Clin N Am 55 (2008) 1343–1358
Garton HJ. Piatt JH. Hydrocephalus. Pediatr Clin N Am 51 (2004) 305– 325
Kusminsky RE. Complications of Central Venous Catheterization. J Am Coll Surg. January 17, 2007.
Marek A. Mirski MA et al. Diagnosis and Treatment of Vascular Air Embolism. Anesthesiology 2007; 106:164–77
Marks JH. Pulmonary Care of Children and Adolescents with Developmental Disabilities. Pediatr Clin N Am 55 (2008) 1299–1314
Munck A et al. Follow-up of 452 totally implantable vascular devices in cystic fibrosis patients. Eur Respir J 2004; 23: 430–434
Shinkwin CA, Gibbin KP. Tracheostomy in children. J Royal Soc Med. Volume 89 April 1996
Simpkins C. Ventriculoperitoneal Shunt Infections in Patients with Hydrocephalus. Pediatr Nurs Nov 2005 Vol 31, No 6
Trachsel D, Hammer J. Indications for tracheostomy in children. Paediatric Resp Rev (2006) 7, 162–168
Wright SE,VanDahm K. Long-term care of the tracheostomy patient. Clin Chest Med 24 (2003) 473– 487