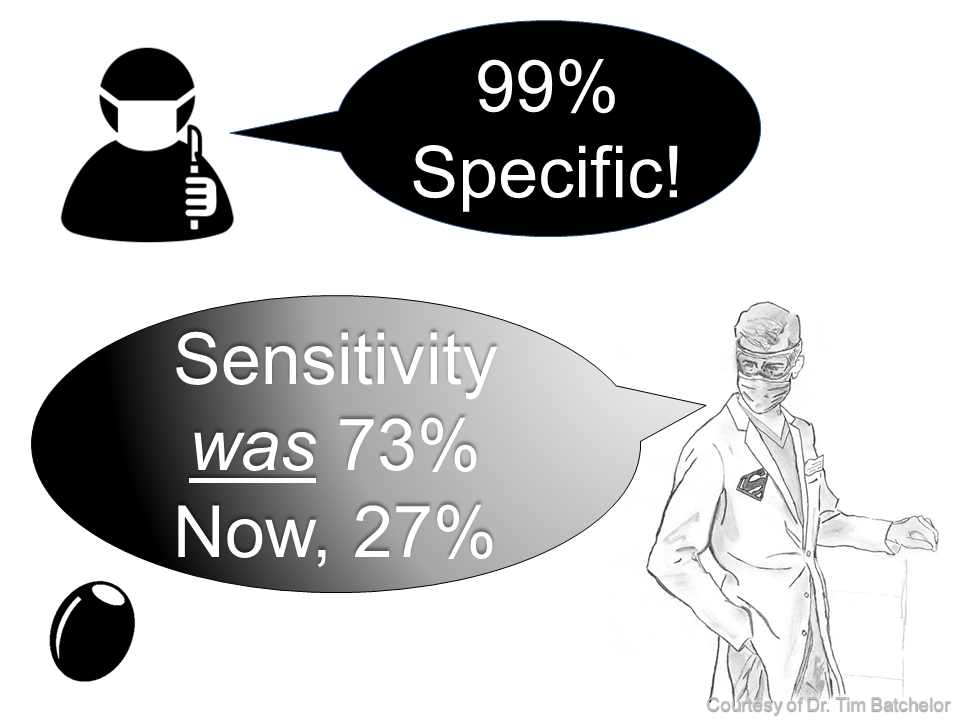
Myth: “No olive, no problem”
Reality: Rare finding, since we diagnose earlier
Pyloric stenosis occurs in young infants because the pyloric sphincter hypertrophies, causing near-complete obstruction of the gastric outlet.
More common in boys, preterm babies, first-born. Less common in older mothers. Association with macrolide use.
Presentation
Young infant arrives with forceful vomiting, but can’t quite get enough to eat “the hungry, hungry, not-so-hippo”.
Early presentation from 3 to 5 weeks of age: projectile vomiting
Later presentation up to 12 weeks: dehydration, failure to thrive, possibly the elusive olive
Labs may show hypOchloremic, hypOkalemic metabOlic acidosis: “all the Os”
Watch out for hyperbilirubinemia, the “icteropyloric syndrome”: unconjugated hyperbilirubinemia from dehydration.
Ultrasound shows a pylorus of greater than 3 mm wide and 14 mm long. Memory aid: 3.14 is “pi”. In pyloric stenosis, π-lorus > 3 x 14
Treatment
Various options, may be deferred depending on age, availability, severity of illness, including:
Pyloromyotomy — definitive. The Ramstedt pyloromyotomy is an open procedure and involves a involves a longitudinal incision along the pylorus, and blunt dissection just to level of the submucosa. The laparoscopic approach (umbilicus) is less invasive but may convey an increased risk of incomplete relief of the obstruction or perforation through the mucosa. Also, this approach involves longer OR and anesthesia time.
Endoscopic balloon dilation – not as effective as pyloromyotomy; reserved for poor surgical candidates.
Conservative management — an NG tube is passed by IR, and the infant slowly feeds and “grows out of it”. Atropine is sometimes used to relax the pyloric sphincter. Also usually reserved for poor surgical candidates.
Selected references
Aboagye J, Goldstein SD, Salazar JH, Papandria D, Okoye MT, Al-Omar K, Stewart D, Lukish J, Abdullah F. Age at presentation of common pediatric surgical conditions: Reexamining dogma. J Pediatr Surg. 2014 Jun;49(6):995-9.
Bakal U, Sarac M, Aydin M, Tartar T, Kazez A. Recent changes in the features of hypertrophic pyloric stenosis. Pediatr Int. 2016 May;58(5):369-71.
Sharp WW, Chan W. Images in emergency medicine. Infant with projectile vomiting. Peristaltic abdominal waves associated with infantile hypertrophic pyloric stenosis. Ann Emerg Med. 2014 Mar;63(3):289,308.
Staerkle RF, Lunger F, Fink L, Sasse T, Lacher M, von Elm E, Marwan AI, Holland-Cunz S, Vuille-Dit-Bille RN. Open versus laparoscopic pyloromyotomy for pyloric stenosis. Cochrane Database Syst Rev. 2021 Mar 9;3(3):CD012827.