Categories
"By the pricking of my thumbs,
Something wheezing this way comes."
-- Witches in Macbeth, with apologies to William Shakespeare
"Bronchiolitis is like a pneumonia you can’t treat.
We support, while the patient heals."
-- Coach, still apologetic to the Bard
The Who
The U.S. definition is for children less than two years of age, while the European committee includes infants less than one year of age.
This is important: toddlerhood brings with it other conditions that mimic bronchiolitis – the first-time wheeze in a toddler may be his reactive airway response to a viral illness and not necessarily bronchiolitis.
The What
The classic clinical presentation of bronchiolitis starts just like any other upper respiratory tract infection: with nasal discharge and cough, for the first 1-2 days. Only about 1/3 of infants will have a low-grade fever, usually less than 39°C. We may see the child in the ED at this point and not appreciate any respiratory distress – this is why precautionary advice is so important in general.
Then, lower respiratory symptoms come: increased work of breathing, persistent cough, tachypnea, retractions, belly breathing, grunting, and nasal flaring. Once lower respiratory symptoms are present, like increased work of breathing, they typically peak at day 3. This may help to make decisions or counsel parents depending on when the child presents and how symptomatic he is.
You’ll hear fine crackles and wheeze. A typical finding in bronchiolitis is a minute-to-minute variation in clinical findings – one moment the child could look like he’s drowning in his secretions, and the next minute almost recovered. This has to do with the dynamic nature of the secretion, plugging, obstruction, coughing, dislodgement, and re-plugging.
The Why
Respiratory syncytial virus is the culprit in up to 90% of cases of bronchiolitis. The reason RSV is so nasty is the immune response to the virus: it binds to epithelial cells, replicates, and the submucosa becomes edematous and hypersecretes mucus. RSV causes the host epithelia and lymphocytes to go into a frenzy – viral fusion proteins turn the membranes into a sticky goop – cells fuse into other cells, and you have a pile-on of multinucleated dysfunction. This mucosal chaos causes epithelial necrosis, destruction of cilia, mucus plugs, bronchiolar obstruction, air trapping, and lobar collapse.
High-Risk Groups
Watch out especially for young infants, so those less than 3 months of age. Apnea may be the presenting symptom of RSV.
Premature infants, especially those less than 32 weeks’ gestation are at high risk for deterioration. The critical time is 48 weeks post-conceptional age.
Other populations at high-risk for deterioration: congenital heart disease, pulmonary disease, neuromuscular disorders, metabolic disorders.
Guiding Principles
In the full term child, greater than one month, and otherwise healthy (no cardiac, pulmonary, neuromuscular, or metabolic disease), we can look to three simple criteria for home discharge.
If the otherwise healthy child one month and older is:
Euvolemic
Not hypoxic
Well appearing
He can likely go home.
The How
Below is a list of modalities, treatments, and the evidence and/or recommendations for or against:
Chest Radiograph
Usually not necessary, unless the diagnosis is uncertain, or if the child is critically ill.
Factors that are predictive of a definite infiltrate are: significant hypoxia (< 92%), grunting, focal crackles, or high fever (> 39°C).
Ultrasound
Not ready for prime time. Two small studies, one by Caiulo et al in the European J or Pediatrics and one by Basile et al. in the BMC Pediatrics that show some preliminary data, but not enough to change practice yet.
Viral Testing
Qualitative PCR gives you a yes or no question – one that you’ve already answered. It is not recommended for routine use. PCR may be positive post-infection for several weeks later (details in audio).
Quantitative PCR measures viral load; an increased quantitative viral load is associated with increased length of stay, use of respiratory support, need for intensive care, and recurrent wheezing. However, also not recommended for routine use.
There is one instance in which viral testing in bronchiolitis can be helpful – in babies less than a month of life, the presence of RSV virus is associated with apnea.
Blood or Urine Testing
Routine testing of blood or urine is not recommended for children with bronchiolitis. Levine et al in Pediatrics found an extremely low risk of serious bacterial illness in young febrile infants with RSV.
The main thing is not to give in to anchoring bias here. If an infant of 3 months of age or older has a clear source for his low-grade fever – and that is his bronchiolitis – then you have a source, and very rarely do you need to go looking any further. He’s showing you the viral waterfall from his nose, and his increased work of breathing. It’s not going to be in his urine.
Bronchodilators!
Should we use bronchodilators in bronchiolitis? It seems lately that this is a loaded question – with strong feelings on either side amongst colleagues. The short answer is that the American Academy of Pediatrics, the UK’s National Institute for Health and Care Excellence, as well as the Canadian Pediatric Society currently recommend against them. However, in continental Europe and Australia, the language is softened to “not routinely recommended”.
Pros and Cons in Audio; the 2006 AAP Guidelines and the 2014 AAP Guidelines use same data to come to divergent recommendations.
Steroids
There is no role for steroids in the treatment of bronchiolitis, even in those with a family or personal history of atopy.
Nebulized Hypertonic Saline
May show some benefit in admitted patients, after repeated treatments; no data to support its use in ED patients (no immediate effect).
Nebulized Epinephrine
One randomized controlled double blinded study in eight centers in Norway published in the NEJM showed no benefit to nebulized epinephrine over nebulized saline. Again, probably asking too much of one single intervention.
The Cochrane review found 19 studies that included a total of 2256 children with acute bronchiolitis treated with nebulized epinephrine. There were no differences in length of hospital stay between the placebo and treatment groups, and so they concluded that for inpatients, nebulized epinephrine is not worth the hassle. However – and this may just be an artifact of meta-analysis – there may be some benefit to outpatients. One study of combined high-dose steroid and epinephrine therapy was not statistically significant when other factors were controlled, but Cochrane concluded that nebulized epinephrine itself may be helpful for outpatients. It won’t affect the overall disease time course, but it may make them feel better enough to go home from the ED and continue observation there.
High-Flow Nasal Cannula Oxygen
High-flow oxygen via nasal cannula requires specialized equipment and delivers humidified oxygen at 1-2 L/g/min. In addition to oxygenation, high flow nasal cannula also likely offers some low-grade positive end-expiratory pressure, which may help with alveolar recruitment. The evidence for its use is based on observational studies, which have found improved respiratory parameters and reduced rates of intubation. Nasal CPAP also has some promising properties in the right clinical setting.
Antibiotics
Not recommended. When bronchiolitis is from a clear viral source, the risk of accompanying bacteremia is less than 1%. A meta-analysis of randomized clinical trials found that antibiotics in bronchiolitis did not improve duration of symptoms, length of hospital stay, need for oxygen therapy, or hospital admission.
Summary: The Good, the Bad, and the Ugly
The Good
Nasal suction and hydration are your best allies. You may elect to give a bronchodilator as a trial once and reexamine, if you’re a bronchodilating believer.
The Bad
Steroids, antibiotics, and a blind obeying of the guidelines. Weigh the risks and benefits of every intervention, including hospitalization – it’s not always a benign thing.
The Ugly
Take a moment to assess the child and make a clinical diagnosis of bronchiolitis, after you’ve excluded cardiac disease, anatomic anomalies, and foreign body aspiration. Wheezing without upper respiratory symptoms is not viral, and it is not bronchiolitis.
When all else fails, remember: in the otherwise healthy, term infant greater than a month of age, if he is well appearing, euvolemic, and not hypoxic, he will often do well with good precautionary advice and supportive care at home. Every thing else: be skeptical, be thorough, and above all, be careful.
Florin TA, Plint AC, Zorc JJ. Viral bronchiolitis. Lancet. 2016 Aug 20. [Epub ahead of print]
Meissner HC. Viral Bronchiolitis in Children. N Engl J Med. 2016 Jan 7;374(1):62-72.
This post and podcast are dedicated to Linda Girgis MD, FAAFP, for her authenticity, innovation, and clear and honest voice on the the frontlines. Thank you, Dr Linda.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
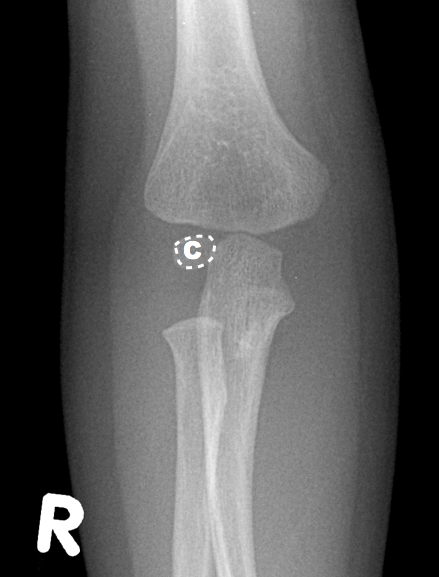
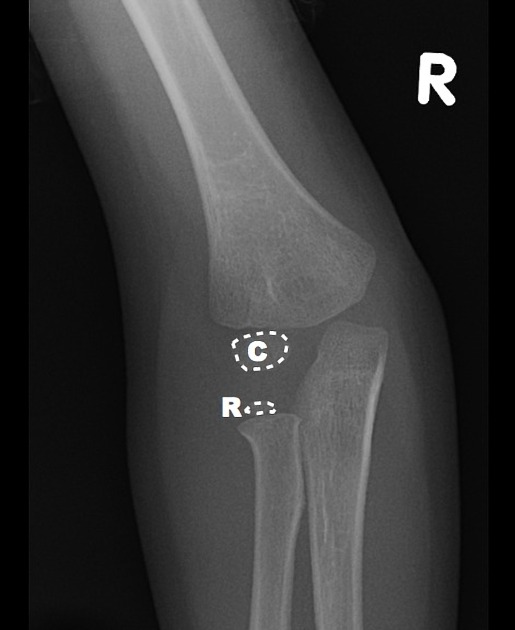
Johnny has fallen on an outstretched hand, and comes to you with a swollen, painful elbow.
Position of comfort, analgesia, xrays, and now what?
What am I seeing -- or not seeing -- here?
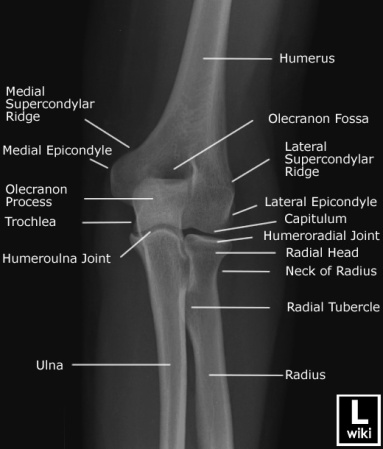
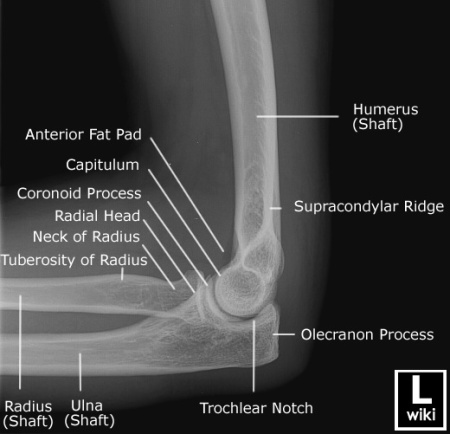
First a refresher on radiographic anatomy of the elbow --
Images courtesy of Radioglypics (Open Access Radiology Education). Used with permission.
Now that we have our adult anatomy reviewed, let's go through the development of the elbow in a child.
We are all born with primary ossification centers -- the basic shapes of our long bones. Secondary ossification centers then develop around the ends of our long bones, and make interpretation of films in the context of suspected injury difficult.
Elbow Interpretation Roadmap: CRITOE
More pragmatic and utilitarian than a prosaic mnemonic, CRITOE helps us to remember the order of ossification of the pediatric elbow.
Although children develop at different rates, the order of ossification is programmed into us. Images courtesy of Radiopaedia.
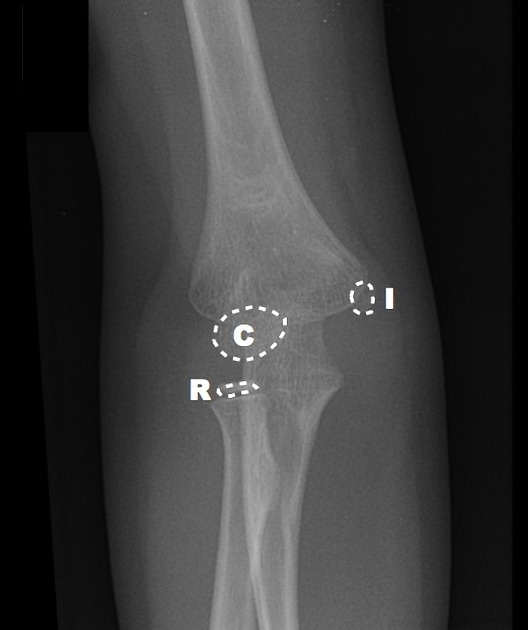
Capitellum
By age one, the capitellum ossifies. On the AP view, imagine a little white oval balloon floating in the darkness between the radius and the humerus.
Radial Head
By age three, the capitellum gets another little balloon to join the party. The radial head is a bony little balloon that floats just above the floor. If you see both little balloons floating on either ends of the space between the humerus and the radius – you know this child is about three years old.
Internal Epicondyle
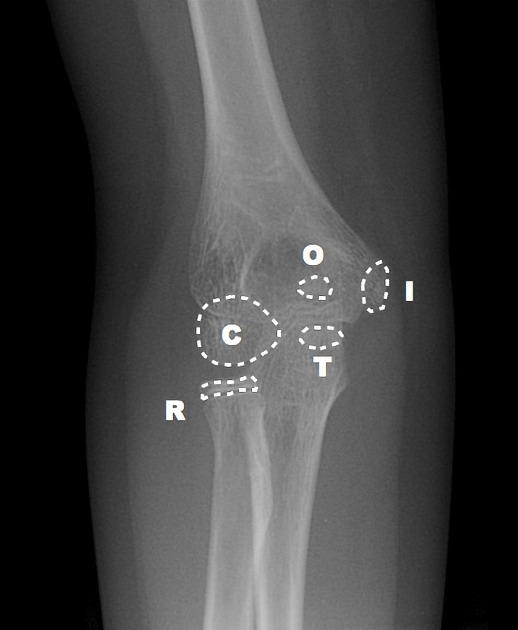
By the age of five, the capitellum and radial head are no longer little floating balloons, but now taking on shapes that resemble what they will look like as an adult. By age five, you’ve grown out of balloons, and have moved on to Frisbees. The internal epicondyle (meaning the medial epicondyle) starts to ossify by age five – a little bony Frisbee.
Trochlea
By age seven, another little Frisbee flies around. On the AP view, the trochlea is superimposed on the humerus – if you look at the distal medial humerus, you’ll see the trochlea like a little oval Frisbee taking shape (see combined film below).
Olecranon
By age nine, the olecranon of the ulna is ossifying. In a nine year old, you’ll see a capitellum, radial head, internal epicondyle, trochlea, and olecranon.
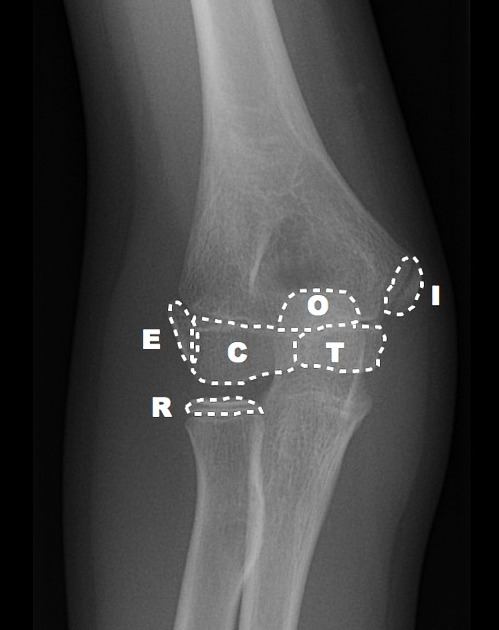
External Epidondyle
By age 11, you start to ossify your external epicondyle (lateral epicondyle).
Pediatric Elbow Films: Putting It All Together
Watch this dynamic video by Dr Jeremy Jones from Radiopaedia:
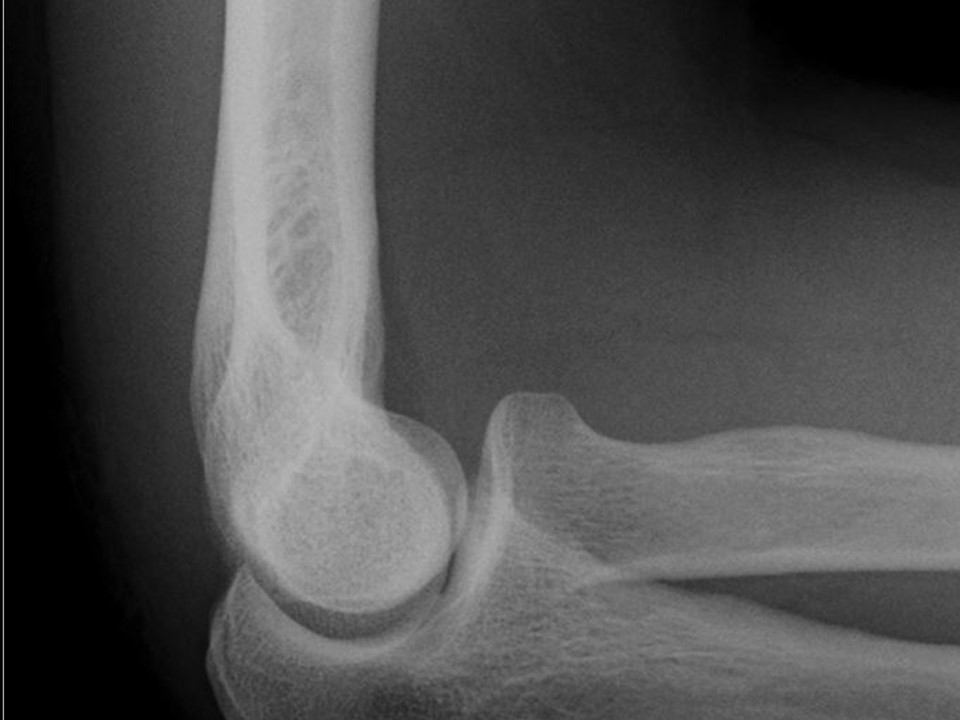
Fracture Saviors: Fat Pads and Drawn Lines
These three things can save us: fat pads, the anterior humeral line, and the radiocapitellar line. Non-annotated images courtesy of Heidi Nunn.
Normal anterior fat pad
Sail sign: billowing hypodensity, indicating blood; sometimes the only (indirect) sign of an elbow fracture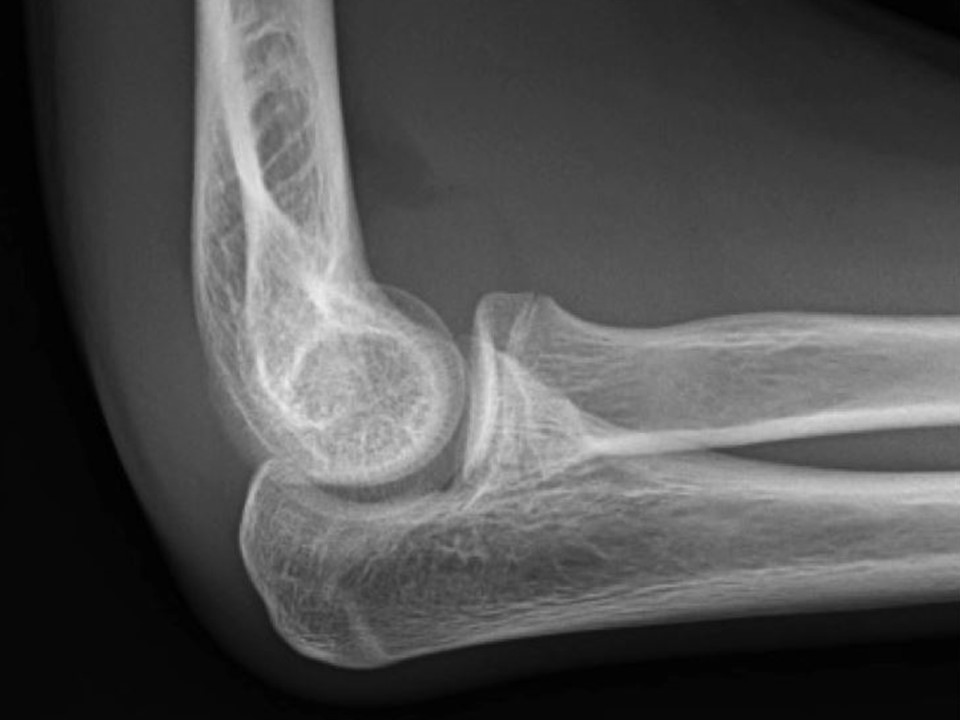
Posterior fat pad: always pathologic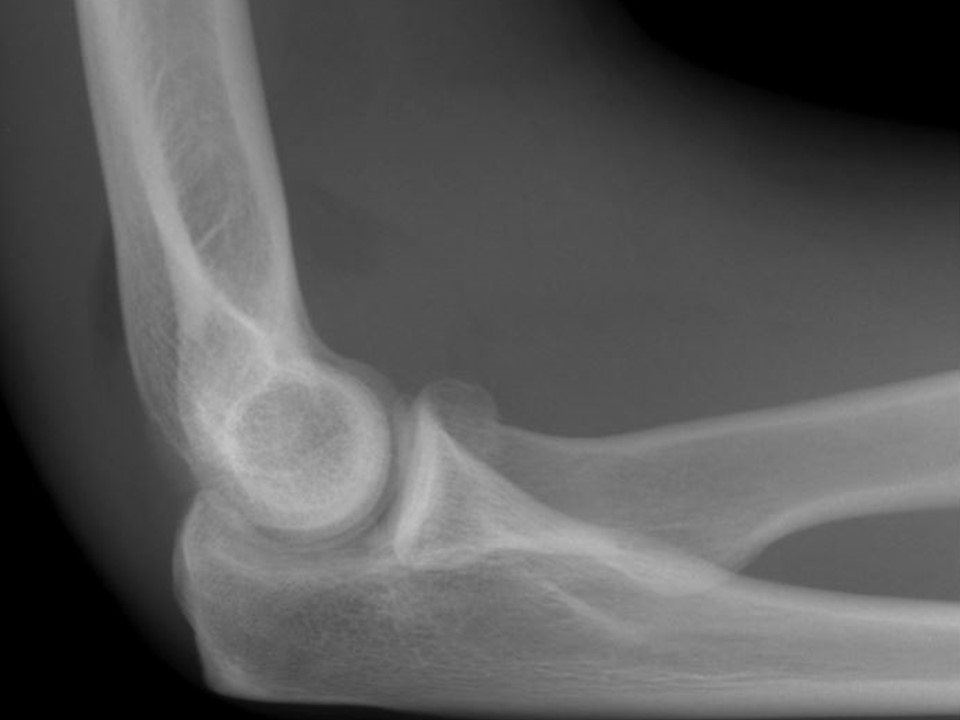
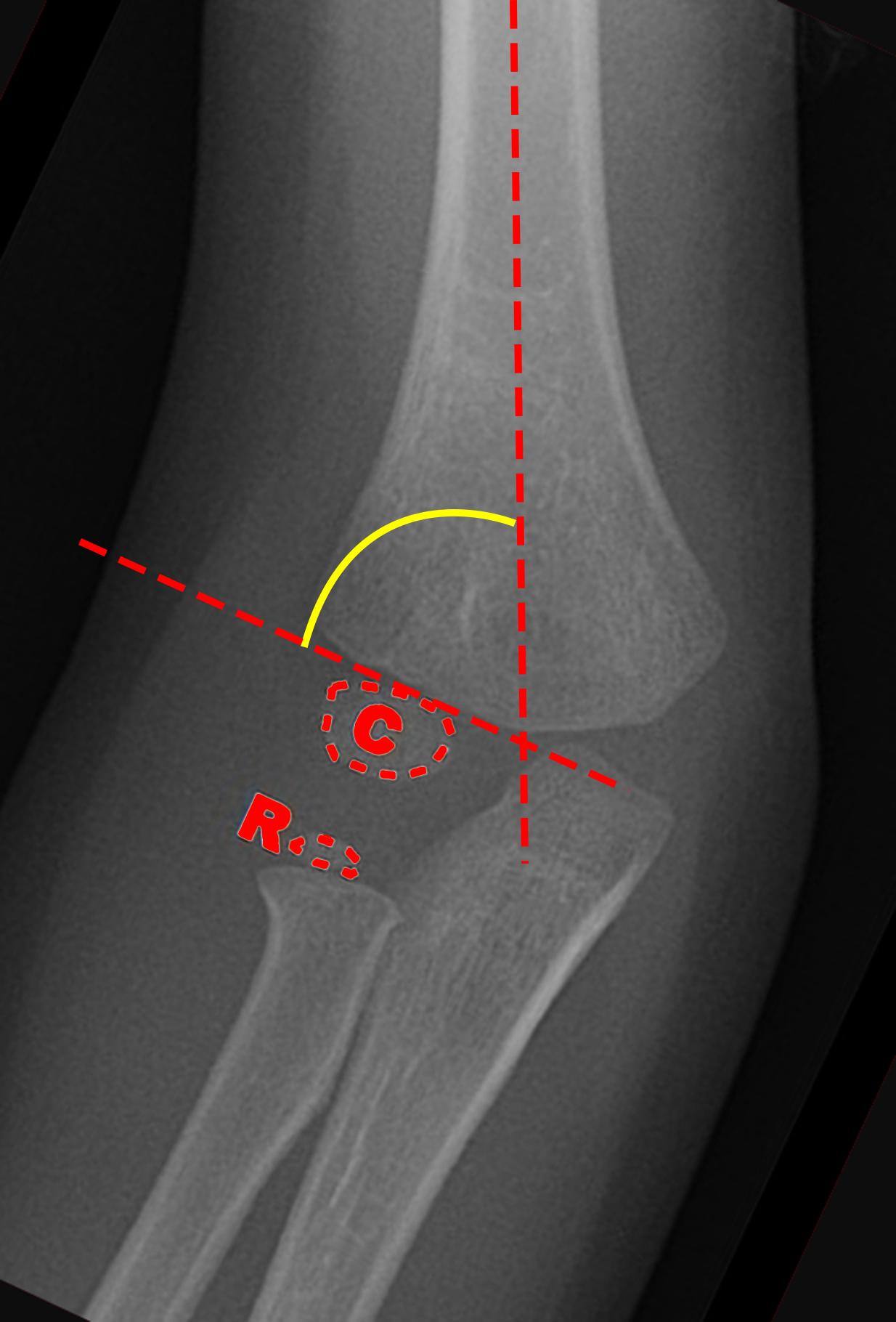
Radiocapitellar Line: anterior humeral line bisects the capitellum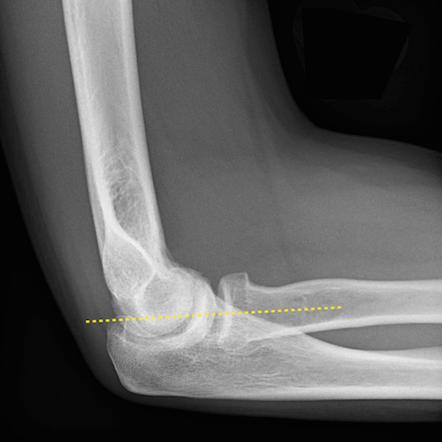
Baumann’s angle (carrying angle): Normal is 70 to 75 degrees. A difference between extremities of just 5 degrees or more is abnormal.
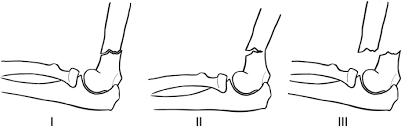
Supracondylar fractures: Gartland Classification
Compartment Syndrome
Pain out of proportion to exam, paresthesias, pallor, poikilothermia, pulselessness, and paralysis
The 6 Ps of compartment syndrome are not sensitive in children.
The only thing that may alert you to increasing compartment pressures in children is an increasing need for analgesics.
Volkmann's ischemic contracture
Untreated compartment syndrome results in thrombosis, edema, ischemia, and disabling contracture.
Other Elbow Injuries
(Details in podcast audio)
Lateral Condyle Fracture
Medial Epicondyle Fracture
Radial head and radial neck fractures
Olecranon fractures
Elbow dislocation
Radial head subluxation (nursemaid’s elbow)
Medial epicondylar apophysitis (Little leager’s elbow)
Test your retention: check out this interactive post from the team at Don't Forget the Bubbles.
Key Points and Summary
The most important pediatric elbow injury is the supracondylar fracture. Grade I is minimally displaced and needs a cast; Grade II is displaced, but with the posterior cortex intact; after closed reduction, the child may still need surgery; Grade III fractures all need closed reduction, internal fixation, and close monitoring for compartment syndrome.
CRITOE gives us the order of ossification for the pediatric elbow – capitellum, radial head, internal epicondyle, trochlea, external epicondyle, and olecranon -- typically occurring at year 1, 3, 5, 7, 9, and 11 – remember the order is the most important thing – all ossification centers should be accounted for. Make sure one is not missing – or where one has been “created” traumatically.
If you don't see the obvious fracture, you can be "saved" by the sail sign and/or a posterior fat pad. Also, make sure to look for the anterior humeral line – on the lateral view, a line drawn down the anterior humerus – if it intersects with the middle third of the capitellum, that is normal – it not, suspect a supracondylar fracture.
The radiocapetellar line runs along the radial neck through the radial head and should line up nicely with the capitellum. If not, assume a fracture-dislocation.
Close communication and coordination with the orthopedist will help us to get the right care at the right time – there is some variability with orthopedic practice, so be open to that – we can make out biggest impact by making the right diagnosis, and aggressively treating pain and effectively providing procedural sedation when needed.
References
Bonus! Watch Larry Mellick Reduce a Nursemaid's Elbow!
https://www.youtube.com/watch?v=-0ROu4hCXwQ
This post and podcast are dedicated to Andy Neill, MBBS. Thank you for your humanism and your dogged dedication to connect with the learner and simplify complex concepts. Welcome back, Andy!
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
Blood in the vomit.
Blood in the stool.
Blood in the diaper.
How far do I go in my investigation?
What do I really have to worry about?
The differential diagnosis of GI bleeding in children is broad.
(Here is the complete differential diagnosis)
In the ED, we can simplify by categorizing by age and appearance. 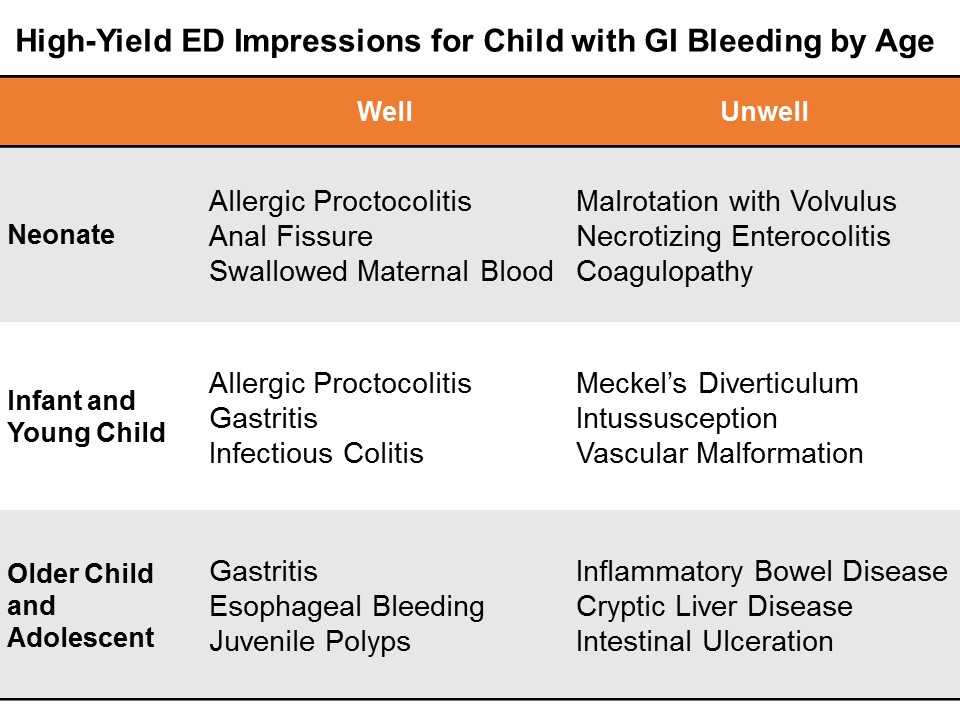
Neonates
GI bleeding in the neonate (less than one month of age) is serious until proven otherwise.
Well appearing?
If this in obvious anal fissure, then no further work-up is necessary. Counsel on proper feeding and follow-up.
Evaluate for potential swallowed maternal blood by examining mother with a chaperone, then perform the Apt test.
Consider allergic proctocolitis if the child is well. Counsel the breastfeeding mother on diet modification. If formula fed, the child should feed through thus until the primary care physician decides whether to start the sticky process of changing up formulas.
If unclear, consider a complete blood count and/or further work-up and admission if unwell.
Ill Appearing?
The three most dangerous diagnoses in the neonate are necrotizing enterocolitis, malrotation with volvulus, and inherited coagulopathy. It is important to note that 15% of necrotizing enterocolitis occurs in full-term babies; malrotation can present simply in shock, without initial overt bleed. Inherited conditions may not be known to the family early on, as they have not yet heard back from the neonatal screening done at birth.
Pitfalls in the neonate and infant
Genitourinary bleeding; hematuria; or uric acid crystals: the classic fake out here is the orange or pink stained diaper – that is actually residue from deposits of uric acid crystals in the urine, an almost always benign phenomenon in which the concentrated crystals oxidize and stain the diaper, frightening the parents.
Think -- pink stain, without clot:

Infants and Young Children
Well appearing?
Through the first year to age 5, things like infectious colitis and gastritis are common.
Ill appearing?
Think about intussusception, cryptic liver disease, or esophageal bleeding. Check the skin – is that a dark purple palpable rash on the buttocks? Think Henoch-Schoenlein purpura.
Focus: Meckel's Diverticulum
Meckel’s diverticulum is the most common congenital malformation of the GI tract, and the most common cause of GI bleeding in the toddler. It is a remnant of the omphalomesenteric tract – it came from a long tube that once connected the yolk sac to the lumen of the midgut. A stranded island of gastric tissue secretes acid in the intestine, where it doesn’t belong. Sometimes these islands never cause much trouble.
When it does present itself, a Meckel’s diverticulum usually follows the rule of twos:
Presents by age 2
Affects 2% of the population
Often 2 inches in length
May include 2 types of mucosa
Found within 2 feet of the ileocecal valve.
Not actively bleeding: technetium-99 pertechnate scintigram (Meckel’s scan).
Actively bleeding: radio-labeled red blood-cell scan (resuscitate and call your surgeons!)
Pitfalls in the infant and young child
Epistaxis; food-related misadventures
Older Child and Adolescent
Well appearing?
Mallory-Weiss tears after forceful vomiting; trivial hemoptysis after viral symptoms; pill esophagitis in the child is just learning to swallow medications. Always consider foreign body ingestion.
Ill Appearing?
Varices from cryptic liver disease; hemorrhagic gastritis; vascular malformation, such as a Dieulafoy lesion, where a tortuous small artery ends just superficial to the gastric mucosa, and can erode through and erupt.
Focus: Inflammatory Bowel Disease
Approximately a quarter of patients with inflammatory bowel disease (IBD) -- both Ulcerative Colitis and Crohn disease – will present by age 20. Children and adolescents may present with the classic symptoms of IBD: abdominal pain, weight loss, bloody diarrhea, but many present atypically with isolated signs like poor growth, anemia, or delayed puberty.
You may also suspect IBD in the child with other extra-intestinal symptoms like oral ulcers, clubbing, erythema nodosum, jaundice, or hepatomegaly.
On history and physical examination, you may get one of three cardinal presentations
Fatigue, history of anemia, in a stable child who comes to the ED with bloody diarrhea
Chronic diarrhea, chronic abdominal pain, and poor weight gain or weight loss
A fulminant presentation, with severe abdominal pain, frankly bloody stools, tenesmus, fever, leukocytosis, and hypoalbuminemia.
On exam, look for general appearance, glossitis from B2 deficiency, hair loss and brittle nails form protein loss, purpura (from vitamin C and vitamin K deficiencies). Look for evidence of episcleritis or uveitis. Listen for rubs as in pericarditis. Do a good abdominal exam, especially looking for hepatomegaly. Perirectal skin tags are not uncommon. Children with IBD may form urinary calculi form oxalate crystal deposition. Do a thorough skin and neurologic exam.
Treatment for both ulcerative colitis and Crohn’s disease is similar.
Induction therapy: children with mild disease get aminosalicylates; those with moderate disease get steroids; and those with severe disease get cyclosporine.
Maintenance regimens to prevent relapse include aminosalicylates, mercaptopurine, and azathioprine.
Surgical treatments for refractory colitis include an ileal pouch and anal anastomosis – also called a J pouch, a type of neorectum created surgically by folding loops of ileum back on themselves and stitching them together to create a larger rectal reservoir where the rectum once was. A neorectum allows the child to have voluntary control of his stools again.
Stabilizing the Pediatric GI Bleed
Life-threatening GI bleeding in children is, thankfully, rare, but we have to be prepared.
Give blood for compensated shock, prepare for massive transfusion if giving more than 40 mL/kg total blood products.
Differing Etiologies: adults and children
The reasons for upper GI bleed in adults are vastly different from those of children. In adults, mostly the life-threats are due to liver disease, varices, or hemorrhagic gastritis.
In children, critical upper GI bleed is often secondary to critical illness (hemorrhagic gastritis or stress ulcer), or vascular malformation. Critical lower gastrointestinal bleeding may be from Meckel's diverticulum or other congenital angiodysplasia.
Endoscopy
Get your patient urgent endoscopy as soon as possible after arrival if there is active bleeding. Otherwise, according to the Belgian guidelines, stable children may have endoscopy within the first 24 hours of hospitalization. The reported efficacy of endoscopy for controlling upper GI bleeding in children is approximately 90%.
Miscellaneous
Nasogastric tube? No routine role (unreliable to rule out or stratify upper GI bleed).
Proton pump inhibitor? No good data, but no major common contraindications.
Octreotide, vasopressin, broad-spectrum antibiotics? May use adult data to extrapolate in the proper etiologic context.
General Advice for the Brisk GI Bleed in Children
This is a rare, but potentially life threatening situation, so anticipate how the child can decline, and get your team assembled: your pediatric intensivist, gastroenterologist, and surgeon – especially if we can’t ge the upper GI bleed to abate with endoscopy. The sooner you activate the team, the better.
Summary
The broad differential diagnosis may be paralyzing, and frustrating, since much of it we cannot discern in the ED.
Consider actionable, high-yield etiologies based on age and appearance.
Neonates
Well appearing? Think rectal fissure, maternal blood, or allergic proctocolitis
Ill appearing? Think necrotizing enterocolitis, malrotation, or an inherited coagulaopathy.
Infants, and Young Children
Well appearing? Think infectious colitis and gastritis.
Ill appearing? Think intussusception and Meckel’s diverticulum.
Older Children and Adolescents
Well appearing? Think Mallory Weiss tear from vomiting, or gastritis
Ill appearing? Think metabolic, cryptic liver disease, or inflammatory bowel disease.
For everyone – a careful history and a good physical exam will point you tto the etiology, or risk-stratify for further outpatient evaluation and management.
References
This post and podcast are dedicated to Carlo D'Apuzzo, MD for his creativity, innovation, and dedication to the highest standards of emergency care. Le tue pillole sono buona medicina. Grazie, Carlo per tutto quello che fai per il mondo #FOAMed.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
Seemingly vague, but potentially dangerous...
common, but possibly with consequences...
...or maybe just plain frustrating.
Let's talk risk stratification, diagnosis, and management.
Primary or Secondary?
We can make headache as easy or as complicated as we like, but let's break it down to what we need to know now, and what the parents need to know when they go home.
Primary headaches: headaches with no sinister secondary cause – like tension or migraine – are of course diagnoses of exclusion (cluster headache is exceedingly rare in children).
Secondary headaches: headaches due to some underlying cause -- are what we need to focus on first.
The list of etiologies is vast; here is just a sampling:
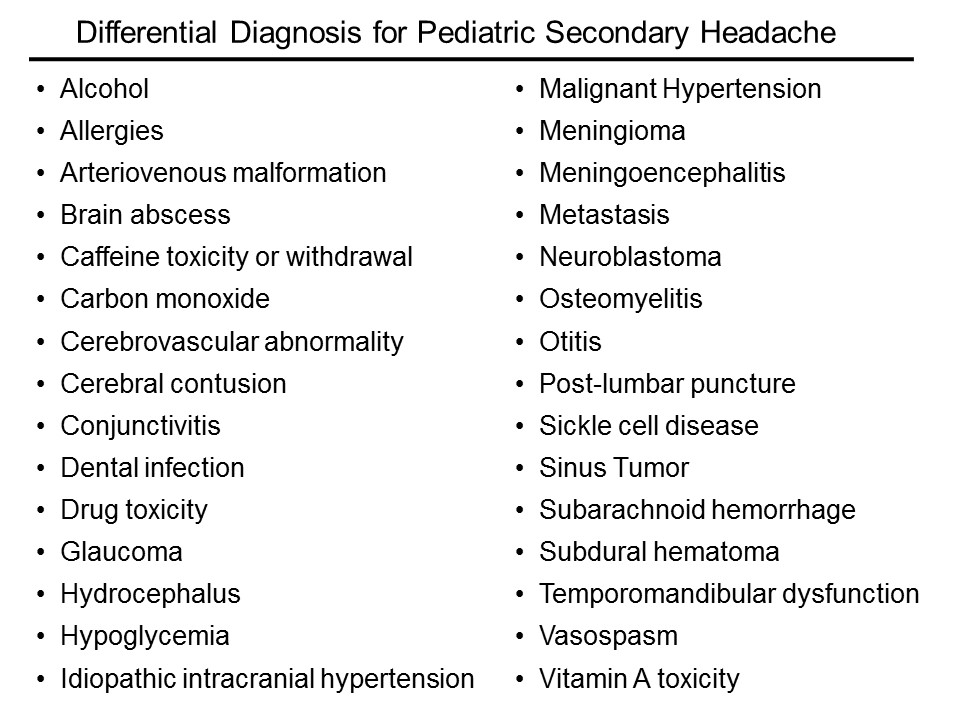
How do I sort this out?
Ask yourself three main questions:
Is it a tumor?
Is it an infection?
Is it a bleed?
Is it a tumor?
Some historical features are high-yield in screening for signs or symptoms consistent with a space occupying lesion.
Progression and worsening of symptoms over time
Associated vomiting
Pain only in the occiput
Headache that is worse with Valsalva – ask if coughing, urinating, or defecating affects the headache
Does this headache wake the child from sleep?
Is it worse in the morning just after getting up?
Conversely, the absence of some historical features may increase suspicion of a space-occupying lesion
No family history of migraine
No associated aura with the headache.
Who needs neuroimaging?
The short answer is, if the child has an abnormal exam finding, then obtain a non-contrast head CT in the ED. If you’re worried enough to get imaging, then you should not feel great about sending him to an expedition to MRI.
The reassuring point is that for a child with a normal neuro exam, we have time to figure this out. For the recurrent headache, outpatient MRI really is the way to go if at all possible – not only do we forgo unnecessary radiation, but MRI is more likely to reveal the cause – or rule out the concern.
Medina et al. in Pediatrics reported on children with headache suspected of having a brain tumor. They stratified patients into low, intermediate, and high risk, based on clinical predictors from the history and physical. All had imaging. They then calculated probability of tumor in each group.
The low risk group had a 0.01% probability of tumor. The intermediate group 0.4%, and the high-risk group had only a 4% probability of tumor. The take-home message is that in the stable patient with a normal neurologic exam and no red flags, time is on our side.
The American Academy of Neurology's most recent guidelines, published first in 1994 and revised in 2004.
1. Neuroimaging on a routine basis is not indicated with recurrent headaches and a normal neurologic exam
2. Neuroimaging should be considered in children with an abnormal exam.
3. Neuroimaging should be considered in children with recent onset of severe headache, change in the type of headache, or associated features that suggest neurologic dysfunction
Is it an infection?
This is nothing new: if you think you need to perform a lumbar puncture, then you’re right. Go after the diagnosis when it meets your threshold for testing.
The difficulty is in the child who just has a headache, plus or minus symptoms that may be viral syndrome.
Dr Curtis et al. in Pediatrics did a systematic review of Clinical Features Suggestive of Meningitis in Children. In the history, only obvious features were helpful in this study: bulging fontanel in the infant or neck stiffness in the older child. Both increased the likelihood of meningitis by 8-fold.
In the physical examination, the only reliable predictors in this study were poor general appearance or a change in behavior.
You will catch those cases, because you would have tuned into meningitis early on -- especially in the unvaccinated.
What about all-comers with fever and headache? The presence of a high fever (so greater than 40 °C) only conferred a positive likelihood ratio of 2.9, only marginally predictive. Reassuring is that for temperatures less than 40 °C, the LR was 1 for meningitis.
In other words, a fever less than 40 °C was just as likely to be present with or without meningitis.
Is it a bleed?
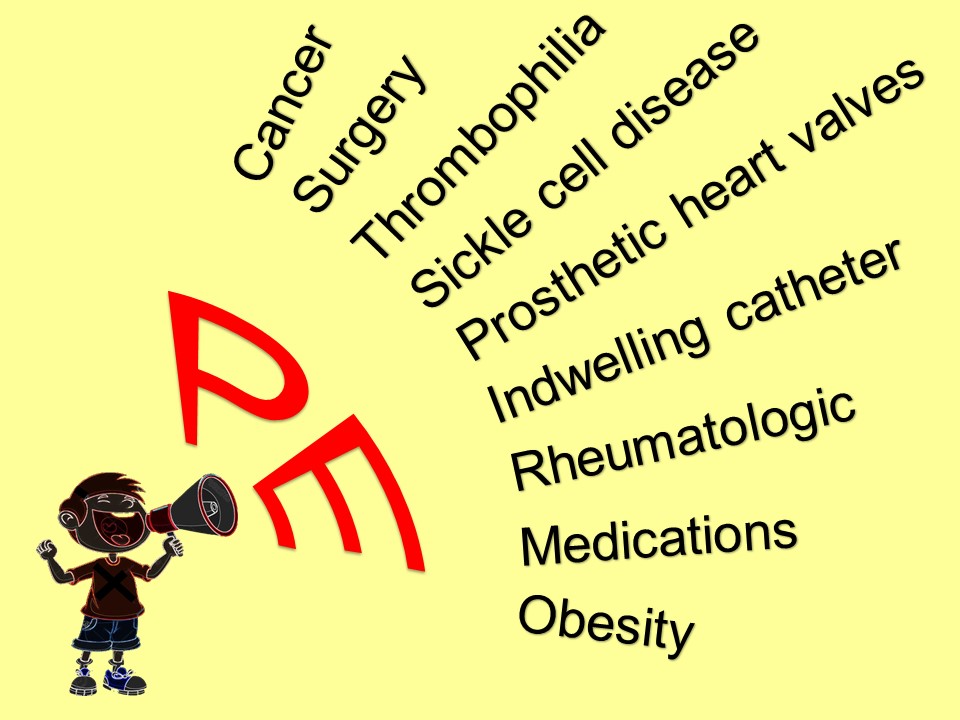
Does this child have some underlying disorder? For example, sickle cell disease, hypertension, rheumatologic disease, or some other endocrine or metabolic disease, such as a mitochondrial disorder?
In chronically ill children, consider cerebral sinus venous thrombosis, vasculitis, ischemia, or hemorrhage.
Arteriovenous malformation (AVM) is the hemorrhage we fear the most.
We really don’t know enough about arteriovenous malformations in the brain to say what is the typical presentation. They may be completely asymptomatic, until they rupture. Even the headache presentation is variable.
Think, headache PLUS.
New headache plus…vomiting.
Headache plus…it’s unilateral and new for the patient.
Headache plus…a new seizure.
Headache plus…focal neuro deficits, that may be transient, due to a vascular steal phenomenon.
Two illustrative cases of arteriovenous malformation:
1. An eleven-year-old girl presents to the ED with new headache, nausea, and vomting in the morning, then had a generalized seizure later that day, and presents with a low GCS. She was intubated, CT confirmed the AVM. She had a right frontal intraparenchymal bleed with midline shift. She underwent clot evacuation and extirpation of the intertwined arteries and veins.
2. A nine-year old girl presented to the ED with headache for two days, constant, then one day of nausea and vomiting. On presentation, she was altered, and had slow-reacting pupils. She also underwent evacuation, and only on histopathology did they find a single, arterialized vein.
Primary Headache: Presumptive Impression
Tension headaches are the most common in children and adults. As in adults, the tension headache is band-like, pressure, tighetening, and often associated with muscle aches in the neck and shoulders.
Find out how often they occur, and whether there is any pattern of worsening symptoms, or if the symptoms seem to be related to sleep hygiene, video games, too much digital screen time. Also, screen for lack of exercise, poor diet, stress, and all of the other good questions you usually ask.
Treat the cause or counsel about lifestyle modification, and offer PO hydration and an NSAID, like ibuprofen or acetaminophen (paracetamol).
Non-pharmacologic techniques like heat packs, rest, stress relief, and a little TLC always help. Be careful not to encourage overreacting to the headache – sometimes we see a pattern of headache, attention, and more headache that can take root. Also look for overuse of medications, which may be the culprit in up to 50% of chronic headaches. Taking NSAIDs 3 or more times per week is associated with medication-induced headache, or cephalalgia medicamentosa.
We often fail to identify migraine headaches in children in the ED, likely for two reasons: prevalence of migraine increases with age, and children don’t present exactly like adults.
Stewart et al. in Neurology, report a prevalence of migraine in children that increases with age: 3 to 7 years of age was 2%; 7 to 11 years of age, 7%; and 11 to 20 years of age, 20%
Pearl: migraines are most commonly bilateral and temporal in children. They resemble "adult" tension headaches, but are much more severe.
We may not be able to sort this out in the ED. The point here is that migraines in children are more common that we may expect, and they can interfere with school performance, with social development, or even with family dynamics and overall stress burden.
Primary Headache Diagnosis: Not (Usually) "Our Thing"
You noticed that we treated before we knew exactly the etiology; such is Emergency Medicine.
We may not be able to make a specific, definitive primary headache diagnosis in the ED, but we should be aware of the criteria to help counsel patients and families.
Tension headache is the most common, but it requires multiple, similar episodes:
Migraine headache (without aura) requires less episodes, but more specific features:
An aura is a fast-pass to diagnosis of migraine:
Primary Headache Management
So how do we treat primary headaches? If you feel this is a mild tension headache, fluids by mouth and a simple NSAID are probably all that is needed, in addition to a heaping dose of reassurance. Ibuprofen (10 mg/kg/dose q 6h, up to 600 mg) for a short course has the most evidence basis. Acetaminophen (paracetamol) (15 mg/kg/dose q6 h) for a short course may also be given.
Abortive treatments with the triptans may have been tried at home, but if they are coming to see us, we are past the point where triptans will be helpful.
For the primary headache that is resistant to NSAIDs, IV therapy may be considered.
If you’re going for IV, a nice evidence-based migraine cocktail is the following:
1. A bolus of 20 ml/kg of normal saline, up to a liter
2. Ketorolac (0.5 mg/kg; max, 30 mg)
3. Diphenhydramine (2 mg/kg; max, 50 mg)
4. Prochlorperazine (0.1 mg/kg; max, 10 mg)
Dr Kaar et al. in Pediatric Emergency Care evaluated the safety and efficacy of their institution’s standardized pediatric migraine practice guideline in the emergency department, which used ths cocktail, based on the best evidence available. In their retrospective chart review, they found the average visual pain scale drop from 7.8 to 2.1
There were no adverse events reported.
So, really you can treat children with migraines very similarly to adults.
Other treatments on the horizon (still under investigation) in children include IV adjuncts such as magnesium, valproic acid, and dexamethasone.
Aftercare and Recurrence Prevention
For everyone who is going home, take just a moment to talk about the importance of sleeping well, eating well, getting exercise, limiting digital screen time, and trying to improve ways of dealing with stress.
When all else fails, and the parent has “heard it all”: get them started on a headache diary.
Take a piece of paper, fold it in half, and start a template for them to work on in a spiral notebook. Start a sample entry for them, with the date and time the headache started, what it felt like, what was happening just before, what made the headache better, any dose of medications given, how long it lasted, and what the patient did after. There are even free apps that will track the headache pattern.
This is the first thing a neurologist will start them on – and it’s sometimes a selling point to the parent that the time spent waiting for a referral to a neurologist is not waste – they will actually be in better shape and can move things along faster. It also gives them some sens of control of what can be a draining situation.
Summary and Mental Road Map
If you were thinking meningitis or acute bleed, especially with fever or meningismus, get a CT first if you see signs of increased intracranial pressure, or if there is an abnormal neuro exam. Otherwise go straight to the lumbar puncture (LP).
In the afebrile child with a normal exam, give symptomatic relief, briefly counsel them, and arrange for follow-up.
In the afebrile child with an abnormal exam, obtain a CT in the ED. If negative, either admit for MRI if you are still concerned, or consider LP for idiopathic intracranial hypertension (pseudotumor cerebri).
Talk with parents early about expectations, and offer them some friendly advice on prevention. Refer patients to the primary care provider or neurologist if the presentation is more involved.
After a good history and physical examination in the ED that results in no red flags, we have time on our side. Help the family through the process by explaining the next steps and what can be done in the meantime. Compassion and a plan: sometimes these are our most powerful allies.
References
This post and podcast are dedicated to Mark Wilson, PhD, BSc, MBBChir, FRCS(SN), MRCA, FIMC, FRGS for his #FOAMed generosity, candor, humility, and dedication to the care of the acutely ill and injured. Thank you.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
Have you ever been in any of these situations?
⇒ You have a stable child who just needs fluids, but no laboratory tests
⇒ You’ve tried PO hydration, to no avail, despite anti-emetics
⇒ You’re poking the stable, but dehydrated child repeatedly without success
What now?
Hypodermoclysis, otherwise known as subcutaneous rehydration.
[Insert Player]
Clysis comes from the same Greek word that “a flood” – hypodermoclysis refers to flooding the subcutaneous space with fluid, so that it can be absorbed systemically.
Sound far-fetched?
Well, it turns out, what is old is new again.
In 1913, Dr Day first described this technique for a child with severe diarrhea who could not tolerate fluids by mouth. Hypodermoclysis then began to gain popularity with a peak of use in the 1940s, until an innovative breakthrough in 1950. Dr David Massa, a resident anesthesiologist at the Mayo clinic, invented the first catheter-over-needle apparatus.
With increasing safety and ready access of IV catheters, IV quickly overshadowed SC.
The subcutaneous route of hydration has also been used effectively in geriatric and palliative care for decades, and it is only now beginning to gain popularity again in its original population: children.
So, how does it work?
In a nutshell, you place a butterfly needle or angiocatheter in the subcutaneous space and you run fluids into it. The tissues quickly absorb the fluids, making them available systemically. That’s it. Everything else is just finesse.
The ideal candidate for hypodermoclysis is the stable patient, with mild to moderate dehydration who fails a trial of fluids by mouth, or who needs a bridge to gaining IV access later, after a slow subcutaneous fluid bolus is given.
Ok, so how do you do it?
Place a topical anesthetic cream, such as EMLA, cover with occlusive dressing (IV dressing), wait 15-20 min
"Pinch an inch" of skin anywhere, but the most practical site in young children is between the scapulae
Insert a 25-gauge butterfly needle or 24-gauge angiocatheter (preferred by the author), secure
Inject 150 U hyaluronidase SC, if available
Infuse 20 mL/kg isotonic solution over one hour, repeat as needed or use "bolus" as bridge to IV access
You can set the line to gravity, and if it is dripping in, you may leave it be. If you see a very slow drip by gravity, or worse, nothing is dripping, you can set the line on a pump, to deliver up to 20 mL/kg over an hour. Infusion at this rate optimizes the balance we want in minimal discomfort while maximizing the flow rate.
This is not a “bolus” in the true sense – but then, when you compare it to the alternative – like IV therapy – and we see a time and cost savings. Dr Mace and colleagues in the American Journal of Emergency Medicine report substantially decreased cost and ED length of stay when comparing the material and human resources needed to place an IV in a squirmy young child, compared with a simple subcutaneous stick.
There will be swelling
There will be swelling – that is the goal. It is really painless, and your patient may lie down on his back with the pump going – it is actually pretty comfortable for most children and adults to do.
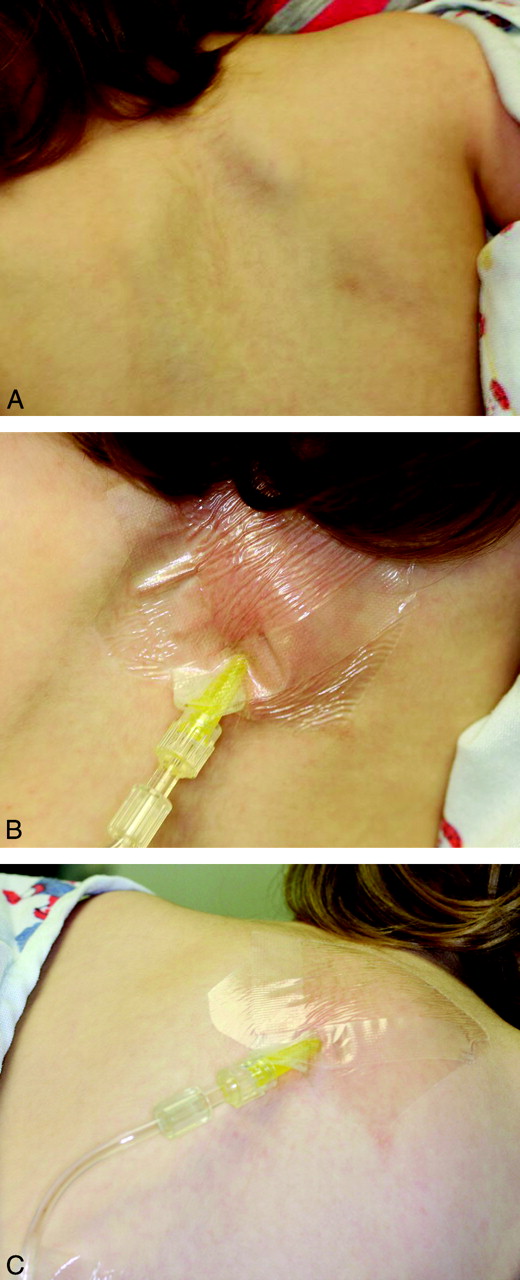 Here’s a tip – since there will be swelling, we want to be careful about how we secure the line, so how you tape it down to the skin is important – we want to avoid a pulling sensation, which can be the beginning of the end of the tolerance for the procedure. Cover that with an occlusive dressing, as you would an IV site. The footprint of the occlusive dressing is relatively small, so it will travel up on top of the subcutaneous mound you’re creating. As the line exits the occlusive patch, place a thin layer of gauze between the skin and the IV tubing, so that the tubing doesn’t press into the skin. Then—as far away from the puncture site as possible—tape it down securely. The idea is not to tape on the growing mound itself, because the mound may pull at the anchored skin and set a nuclear chain reaction of annoyance and restlessness – and potentially a failed procedure.
Here’s a tip – since there will be swelling, we want to be careful about how we secure the line, so how you tape it down to the skin is important – we want to avoid a pulling sensation, which can be the beginning of the end of the tolerance for the procedure. Cover that with an occlusive dressing, as you would an IV site. The footprint of the occlusive dressing is relatively small, so it will travel up on top of the subcutaneous mound you’re creating. As the line exits the occlusive patch, place a thin layer of gauze between the skin and the IV tubing, so that the tubing doesn’t press into the skin. Then—as far away from the puncture site as possible—tape it down securely. The idea is not to tape on the growing mound itself, because the mound may pull at the anchored skin and set a nuclear chain reaction of annoyance and restlessness – and potentially a failed procedure.
The swelling will look indurated, a pinkish red. It’s not an allergic reaction: even with the old preparations of hyaluronidase, allergic reactions were rare, and now they are very rare with the recombinant preparation. It is supposed to swell and look ugly. The subcutaneous tissues will swell to a point where you have a steady state fluid administration rate, and as soon as you stop the infusion, the remaining fluid will start to subside as it is absorbed.
A Bridge to IV Therapy?
Kuensting et al. in the Journal of Emergency Nursing in 2013 compared subcutaneous fluid infusion with intravenous fluid infusion in children with difficult IV access. They found the mean time from order entry to subcutaneous fluid infusion to be 20 min, compared to the failed IV access group with an average infusion start time of 1.5 hours. The latter group eventually received subcutaneous fluids. The investigators also found a shorter ED length of stay in the subcutaneous group.
In the same study, a subgroup received subcutaneous fluids initially, and later an IV. They found a trend in ease of IV access after subcutaneous fluid therapy. In other words, if your little patient with difficult IV access is hemodynamically stable and amenable to a bolus over an hour, you may choose to start with hypodermoclysis and reevaluate.
Predicting Difficult IV Access in Children
Much has been studied and written about the predictors of difficult IV access in children. The most often cited are: age < 3 years, weight less than 5 kg, prematurity, obesity, and darker skin tones, where the contrast of vein to skin may not be so apparent.
The three main predictors of the score validated by Riker et al. in Annals of Emergency Medicine include the most practical and universal of features: vein palpability, vein visibility, and patient age.
If you’re anticipating difficult IV access in the child who can stand to wait an hour for a slow bolus, you may start with the subcutaneous route to get those veins plumper and more visible, to improve your chances of IV access in the very near future.
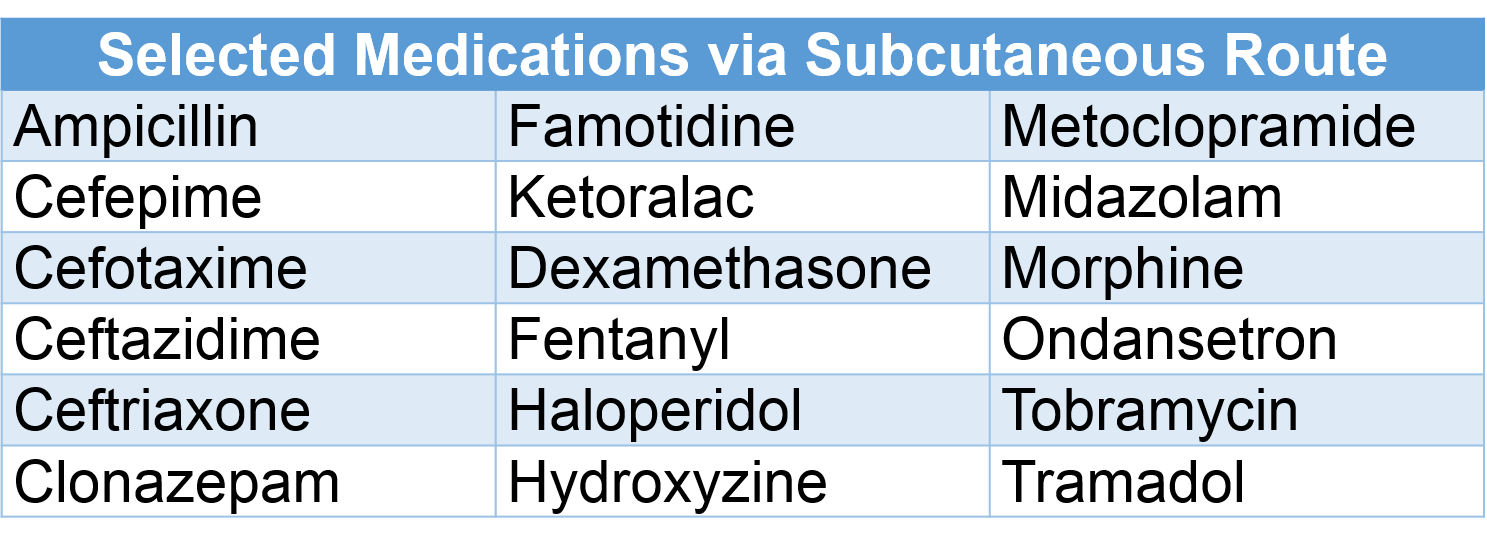
Medications via Subcutaneous Route
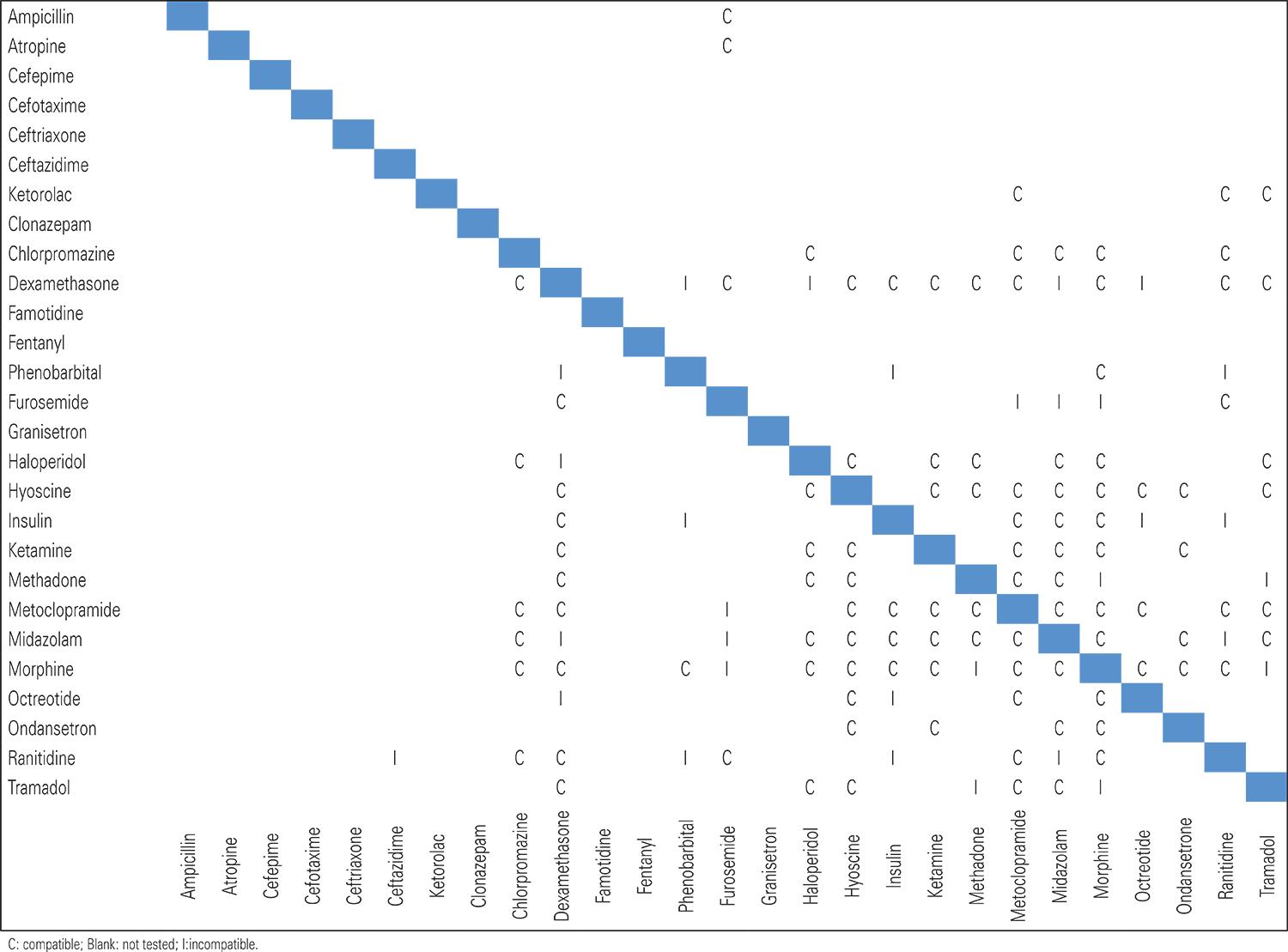
Certain medications have been used safely via subcutaneous infusion; always check dose, rate, and compatibility.
What about catheter size?
You don’t need to use larger needles or angiocathters for older children, adolescents or adults. A 25-gauge butterfly or 24-gauge angiocatheter works well from an infant to an elder. In one study of adults, a half a liter of saline was infused by gravity via a 24-gauge catheter. With IVs, the shorter and larger the bore, the faster the infusion.
In subcutaneous infusion, it is not the size of the catheter, but the osmotic gradient that determines the rate of absorption.
What if I don't have that fancy hyaluronidase?
It’s actually increasingly readily found – and available in generic form. If you have it, please use it – it will make a believer out of you and others.
Hypodermoclysis will work without hyaluronidase – the process of subcutaneous rehydration just takes a lot longer to work. In a double-blind cross-over trial Thomas et al. in 2007 compared subcutaneous administration of lactated ringer’s solution by gravity with and without hyalurondase. The hyaluronidase group received their fluids 5 times faster. The average rate of the hyaluronidase group was 382 mL/h versus the fluid only group, who did not receive hyalurinodase; they were substantially slower, at 82 mL/h. It’s worth using if you have it, but still potentially useful if you don’t.
Recap: Supplies
√ EMLA or any topical anesthetic used for intact skin, placed as soon as the decision is made
√ A 25-gauge butterfly needle or 24-gauge angiocatheter
√ IV tubing, gauze to pad, tape to anchor
√ 150 U hyaluronidase, the same dose, regardless of age or size
√ Isotonic fluids – you can start with 20 ml/kg
√ And finally a well informed team made up by the patient, the parents, and your staff, so that everyone knows what to expect for a successful subcutaneous fluid administration.
References
Smith LS. Hypodermoclysis with older adults. Nursing. 2014 Dec;44(12):66.
This post and podcast are dedicated to Christina L. Shenvi, MD, PhD, for her dedication to excellence in patient care and enthusiasm in #FOAMed, Emergency Medicine, and Geriatric Emergency Medicine. There are many shared lessons learned in the care of children, elders, and families. Thank you.
Catch Dr Shenvi on the innovative GEMcast.
Subcutaneous Infusion
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
"She won't walk", or "He just looks like he's limping".
So many things can be going on -- how do we tackle this chief complaint?
You’re dreading a big work-up. You almost want to tell the kid – please, STOP LIMPING...
STOP LIMPING!
S – Septic Arthritis
The most urgent part of our differential diagnosis. The hip is the most common joint affected, followed by the knee. Lab work can be helpful, as well as US of the hip to look for an effusion, but sometimes, regardless of the results, the joint just has to be tapped to know for sure.
T – Toddler’s fracture
This is usually a torque injury when the wobbling toddler pivots quickly or trips and falls. Toddler’s fractures happen in children 1 to 3 years of age, and occur in the distal 1/3 of the tibia. Sometimes a cast is needed, but currently there is a new trend in foregoing casting in mild cases.
O – Osteomyelitis
Bacteremia – from any source – can seed into any bone. It’s not very common, but it happens: approximately 2% of children who present to an ED with limp will have osteomyelitis. Plain films, ESR, and CRP are a fair screen to start. For more than the casual concern, MRI is the best modality to evaluate, followed by radionuclide scintigraphy. Although not the first choice modality, CT can show periosteal changes, such as inflammatory new bone formation or periosteal purulence.
P – Perthes disease
This is the famous Legg-Calvé-Perthes idiopathic avascular necrosis of the hip, usually affecting children from 3 to 12 years. They present with a slow onset pain and with an antalgic gait. Patients will have trouble with internal rotation and abduction of the hip. Radiographs may be initially normal. MRI can show the culprit: decreased perfusion to the femoral head and subsequent necrosis.
L – Limb-Length Discrepancy
Parents may notice that he seems “wobblier” than he should be. It may be that we are just now appreciating a congenital anomaly. Get out the paper tape, and measure from the anterior superior iliac spine to the medial malleolus and compare both sides. Children with limb-length discrepancy only need a non-urgent referral to pediatric orthopedics to look for congenital dysplasia of the hip, or other growth abnormalities. Some are treated with orthotics. Surgical options vary. Epiphysiodesis destroys the growth plate on the unaffected side, which evens out the growth. Other options are limb-lengthening or limb-shortening procedures.
I – Inflammatory
Transient Synovitis. This is what we want them to have right? The typical age is between 3 and 6 years, sometimes just after a URI. To be comfortable with this diagnosis, we should have considered all of the dangerous diagnoses, the child should be well, afebrile, in minimal discomfort, and he should respond almost completely to an NSAID. He’s the one running up and down the department after treatment – or just from sheer boredom after observation.
M – Malignancy
Primary bone tumors such as Ewing’s sarcoma or osteogenic sarcoma typically affect older children. Limping, however, may be a presenting symptom of leukemia. If you have any suspicion of the general wellness of the child, get a screening CBC, and perhaps a peripheral blood smear. Whatever you do, make sure you get close follow up for these kids that are on your malignancy radar -- the blast crisis may not have occurred yet – but it can happen hours to days later.
Plain films are insensitive for leukemic involvement of bone but they may show diffuse osteopenia, or metaphyseal bands – symmetrical high-uptake markings around the joint. They look like stacks of paper within normal bone – you can see them also in anemia, lead poisoning, and other causes. Also look for periosteal new bone formation, sclerosis, or lysis.
P – Pyomyositis
This usually presents with vague irritability, pain, and fever, and sometimes with a subacute minor trauma. These children don’t look to well.
Also think about just run-of-the-mill myositis, usually from a viral cause, such as influenza. Typically the calves are affected and are always tender. Hydration and supportive therapy are indicated for viral causes.
For bacterial focal pyomyositis, give empiric antibiotics, admit them for major inpatient workup, and think about early surgical consultation if you think you need sepsis source control.
I – Iliopsoas Abscess
Children most often will develop a primary abscess from bacteremia from an unresolved infection. Adults more commonly form secondary abscesses from Crohn’s disease, post-op complications, a vertebral infection, or even a bad chronic urinary tract infection. Lest you think this is a dramatic presentation, think again: iliopsoas abscesses present also with vague symptoms of back, flank, abdominal, or hip pain, sometimes with fever. The median time from symptoms to diagnosis in children is a whopping 20+ days, according to one study. If iliopsoas abscess is starting to get your attention, get the CT or MRI.
N – Neurologic
Not to scare you, but children do have strokes; unlike adults, half are hemorrhagic, half are thromboembolic. Typically they’ll have some underlying pathology that will alert you, such as a cardiac lesion, sickle cell disease, or some infectious or metabolic history. The good news is that it won’t just be a limp – you’ll have some other neuro sign or symptom to go after.
Guillain Barré is another thing to consider – early lower extremity weakness may present as a limp or refusal to walk. Maybe it’s not the hip that should be tapped, but the spinal canal.
Think also about muscular dystrophy or peripheral neuropathy and its possible underlying etiology.
G – Gastrointestinal and Genitourinary
What else could be going on? Appendicitis may be faking you out here. Perhaps there is a hernia, or testicular or ovarian torsion, all of which can present as lateralizing pain and not wanting to walk. Think outside the box.
Phases of Gait
The gait cycle has three phases: contact, stance, and propulsion. Contact is the time from heel strike to just when the foot is flat. Stance is from the foot being flat to lifting the heel from the ground. The stance phase is when you bear most of your weight. The propulsion phase is when your weight transfers to your toes, and you push off.
Abnormal Gaits
Antalgic Gait -- "hobbling" gait; normal contact phase, but stance phase is abbreviated; propulsion is normal. The patient is trying to limit the time spent bearing weight on that side.
Trendelenburg Gait -- the affected side's hip abductor muscles are too weak or painful to stabilize the pelvis; the unaffected side dips to the floor. May be superior gluteal neuropathy, or a biomechanical problem, such as avascular necrosis, congenital dysplasia of the hip, or slipped capital femoral epiphysis.
Circumduction Gait -- the patient swings his foot laterally (due to a foot or ankle pathology), or to avoid tripping in limb-length discepancy.
Stiff-leggged Gait -- the patient walks with knees locked, in an attempt to avoid using the gastrocnemius muscles; concerning for myositis.
Equinus Gait -- toe-walking, as seen in myositis, also to avoid exacerbating pain from the calves.
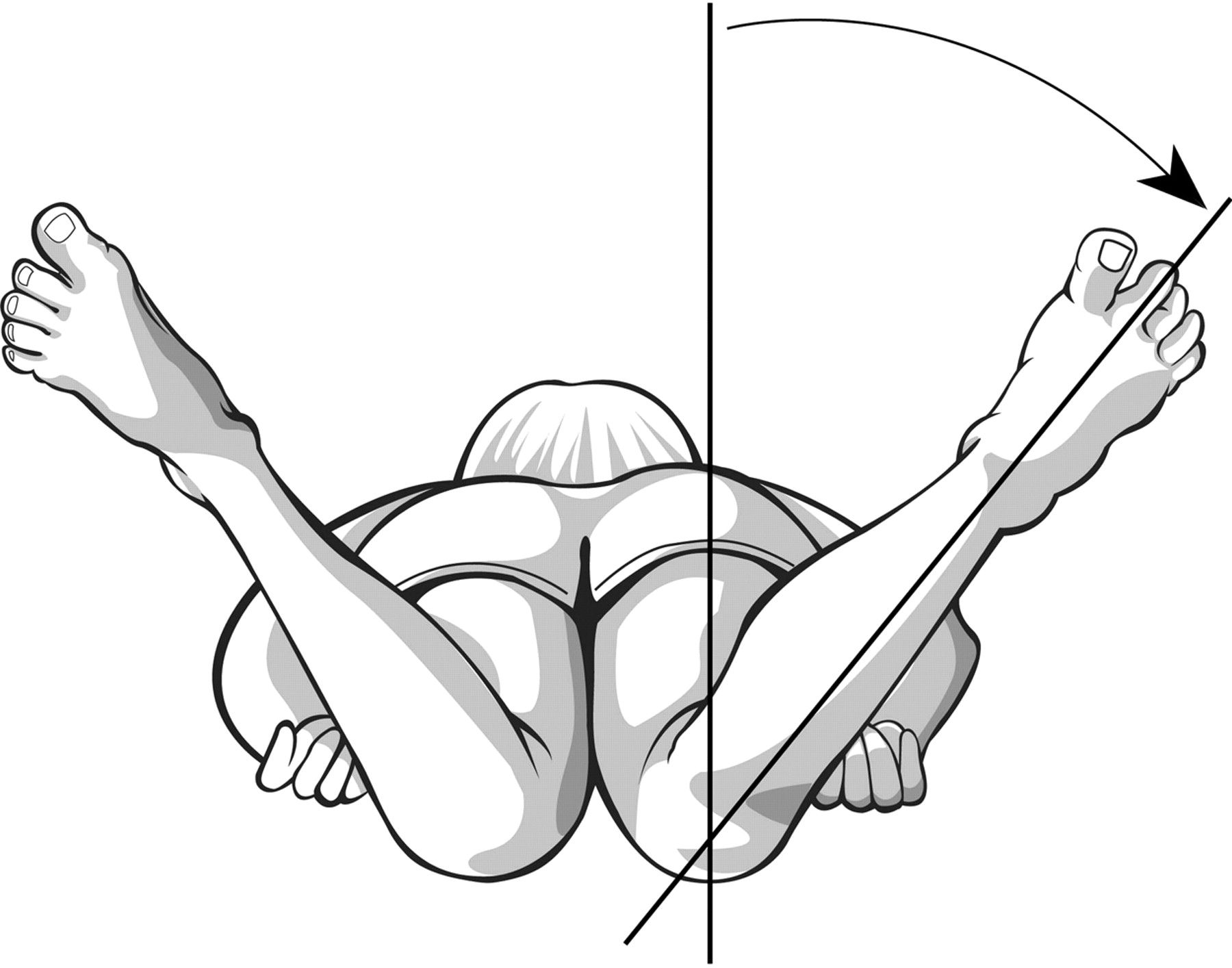 Lag of Internal Rotation of the Hip
Lag of Internal Rotation of the Hip
Look for symmetry of internal rotation, or lateralizing pain or "guarding" with range of motion.
Keep the pelvis flush to the bed, and simultaneously rotate the lower extremity laterally, which will cause internal rotation of the hip.
Avascular necrosis will not allow full internal rotation, since the joint space is narrowed with this maneuver, causing impingement of the sensitive necrotic head of the femur.
Note any pain, asymmetry, and angle of internal rotation achieved.
Kocher Criteria
In their original paper in 1999, Dr Kocher et al. performed a retrospective analysis of children who were being evaluated for a septic joint versus transient synovitis over a 15 year period, in a major referral center. They came up with four independent predictors of a septic joint, and calculated the probability of septic arthritis based on the number of features present. In 2004 the same group validated their prediction tool, with a slightly decreased sensitivity and specificity in the validation population.
In short, the Kocher criteria are not perfect, but it’s the best evidence we have at the moment.
The four predictors are:
Inability to walk
Fever of 38.5 C of greater
ESR > 40 mm/h
WBC > 12,000
Bonus mnemonic: Walk FEW: Inability to Walk | Fever | ESR | WBC
The probability of septic arthritis increases with increasing predictor. In this prediction model, each predictor has the same weight.
Probability of Septic Arthritis (Kocher et al. 1999)
0 Predictor – <0.2 %
1 Predictor – 3%
2 Predictors – 40%
3 Predictors – 93.1%
4 Predictors – 99.6%
Now, remember, this is to be used in children in whom you already have some suspicion of a septic joint. So, 0 predictors, generally you’re alright. 1 predictor, you may start to worry. Once you have 2 predictors, your chances jump for 3% to 40%. You really have to go looking.
The Kocher caveat is that there is no single test or single decision rule that will stop you from investigating if you are concerned enough. Don’t have too much faith in this imperfect decision tool – we use it because we need somewhere to start. Treat and push for the aspiration of the hip if you are left in doubt. Septic arthritis can be devastating if not identified early.
Summary
- Fever and limp? – do not pass go – especially with 2 or more Kocher criteria – or if there is any doubt, tap that joint.
- Refusal to walk after adequate analgesia? Admit for observation, MRI, further workup, blood cultures, and much more.
- Remember some developmental concerns to help you to decide whether to continue the investigation urgently as an inpatient, or non-urgently as an outpatient.
- After all of that, try to get them them to STOP LIMPING!
References
Lindsay D, D'Souza S. A limping child. BMJ. 2016 Feb 9;352:i476.
Verma S. Pyomyositis in Children. Curr Infect Dis Rep. 2016 Mar;18(4):12.
This post and podcast are dedicated to the estimable yet graciously humble Andrew Tagg BSC(Hons), MBBS, MRCSEd, ACEM for his dedication to #FOAMed, Emergency Medicine, Pediatric Emergency Medicine, and all things caffeinated. Thank you for your dedication, generosity, and your example.
Don't Forget the Conference! #DFTB17 #DFTB
Do we recognize shock early enough?
How do we prioritize our interventions?
How can we tell whether we’re making our patient better or worse?
World wide, shock is a leading cause of morbidity and mortality in children, mostly for failure to recognize or to treat adequately.
So, what is shock?
Simply put, shock is the inadequate delivery of oxygen to your tissues. That’s it. Our main focus is on improving our patient’s perfusion.
Oxygen delivery to the tissues depends on cardiac output, hemoglobin concentration, the oxygen saturation of the hemoglobin you have, and the environmental partial pressure of oxygen.
At the bedside, we can measure some of these things, directly or indirectly. But did you notice that blood pressure is not part of the equation? The reason for that is that blood pressure is really an indirect proxy for perfusion – it’s not necessary the ultimate goal.
The equation here is a formality:
DO2 = (cardiac output) x [(hemoglobin concentration) x SaO2 x 1.39] + (PaO2 x 0.003)
Shock CAN be associated with a low blood pressure, but shock is not DEFINED by a low blood pressure.
Compensated Shock: tachycardia with poor perfusion. A child compensates for low cardiac output with tachycardia and a increase in systemic vascular resistance.
Decompensated Shock: frank hypotension, an ominous, pre-arrest phenomenon.
Shock is multifactorial, but we need to identify a primary cause to prioritize interventions.
How they "COHDe": Cardiogenic, Obstructive, Hypovolemic, and Distributive.
Cardiogenic Shock
All will present with tachycardia out of proportion to exam, and sometimes with unexplained belly pain, usually due to hepatic congestion. The typical scenario in myocarditis is a precipitous decline after what seemed like a run-of-the-mill URI.
Cardiogenic shock in children can be from congenital heart disease or from acquired etiologies, such as myocarditis. Children, like adults, present in cardiogenic shock in any four of the following combinations: warm, cold, wet, or dry.
"Warm and Dry"
A child with heart failure is “warm and dry” when he has heart failure signs (weight gain, mild hepatomegaly), but has enough forward flow that he has not developed pulmonary venous congestion. A warm and dry presentation is typically early in the course, and presents with tachycardia only.
"Warm and Wet"
If he worsens, he becomes “warm and wet” with pulmonary congestion – you’ll hear crackles and see some respiratory distress. Infants with a “warm and wet” cardiac presentation sometimes show sacral edema – it is their dependent region, equivalent to peripheral edema as we see in adults with right-sided failure.
“Warm” patients – both warm and dry and warm and wet -- typically have had a slower onset of their symptoms, and time to compensate partially. Cool patients are much sicker.
"Cold and Dry”
A patient with poor cardiac output; he is doing everything he can to compensate with increased peripheral vascular resistance, which will only worsen forward flow. Children who have a “cold and dry” cardiac presentation may have oliguria, and are often very ill appearing, with altered mental status.
"Cold and Wet"
The sickest of the group, this patient is so clamped down peripherally that it is now hindering forward flow, causing acute congestion, and pulmonary venous back-up. You will see cool, mottled extremities.
Cardiogenic Shock: Act
Use point-of-care cardiac ultrasound:
Good Squeeze? M-mode to measure fractional shortening of the myocardium or anterior mitral leaflet excursion.
Pericardial Effusion? Get ready to aspirate.
Ventricle Size? Collapsed, Dilated,
Careful with fluids -- patients in cardiogenic shock may need small aliquots, but go quickly to a pressor to support perfusion
Pressor of choice: epinephrine, continuous IV infusion: 0.1 to 1 mcg/kg/minute. Usual adult starting range will end up being 1 to 10 mcg/min.
Avoid norepinephrine, as it increases systemic vascular resistance, may affect afterload
Just say no to dopamine: increased mortality when compared to epinephrine
Obstructive Shock
Mostly one of two entities: pulmonary embolism or cardiac tamponade.
Pulmonary embolism in children is uncommon – when children have PE, there is almost always a reason for it – it just does not happen in normal, healthy children without risk factors.
Children with PE will either have a major thrombophilic comorbidity, or they are generously sized teenage girls on estrogen therapy.
Tamponade -- can be infectious, rheumotologic, oncologic, or traumatic. It’s seen easily enough on point of care ultrasound. If there is non-traumatic tamponade physiology, get that spinal needle and get to aspirating.
Obstructive Shock: Act
Pulmonary embolism (PE) with overt shock: thrombolyse; otherwise controversial. PE with symptoms: heparin.
Tamponade: if any sign of shock, pericardiocentesis, preferentially ultrasound-guided.
Hypovolemic Shock
The most common presentation of pediatric shock; look for decreased activity, decreased urine output, absence of tears, dry mucous membranes, sunken fontanelle. May be due to obvious GI losses or simply poor intake.
Rapid reversal of hypovolemic shock: may need multiple sequential boluses of isotonic solutions. Use 10 mL/kg in neonates and young infants, and 20 mL/kg thereafter.
Hypovolemic Shock: Act
Tip: in infants, use pre-filled sterile flushes to push fluids quickly. In older children, use a 3-way stop cock in line with your fluids and a 30 mL syringe to "pull" fluids, turn the stop cock, and "push them into the patient.
Titrate to signs of perfusion, such as an improvement in mental status, heart rate, capillary refill, and urine output.
When concerned about balancing between osmolality, acid-base status, and volume status, volume always wins. Our kidneys are smarter than we are, but they need to be perfused first.
Distributive Shock
The most common cause of distributive shock is sepsis, followed by anaphylactic, toxicologic, adrenal, and neurogenic causes. Septic shock is multifactorial, with hypovolemic, cardiogenic, and distributive components.
Children with sepsis come in two varieties: warm shock and cold shock.
Distributive Shock: Act
Warm shock is due to peripheral vascular dilation, and is best treated with norepinephrine.
Cold shock is due to a child’s extreme vasoconstriction in an attempt to compensate. Cold shock is the most common presentation in pediatric septic shock, and is treated with epinephrine.
Early antibiotics are crucial, and culture everything that seems appropriate.
Shock: A Practical Approach
"How FAST you FILL the PUMP and SQUEEZE"
Sometimes things are not so cut-and-dried. We'll use a practical approach to diagnose and intervene simultaneously.
Look at 4 key players in shock: heart rate, volume status, contractility, and systemic vascular resistance.
How FAST you FILL the PUMP and SQUEEZE
First, we look at heart rate -- how FAST?
Look at the heart rate – is it sinus? Could this be a supraventricular tachycardia that does not allow for enough diastolic filling, leading to poor cardiac output? If so, use 1 J/kg to synchronize cardiovert. Conversely, is the heart rate too slow – even if the stroke volume is sufficient, if there is severe bradycardia, then cardiac output -- which is in liters/min – is decreased. Chemically pace with atropine, 0.01 mg/kg up to 0.5 mg, or use transcutaneous pacing.
If the heart rate is what is causing the shock, address that first.
Next, we look at volume status.
How FAST you FILL the PUMP and SQUEEZE
Look to FILL the tank if necessary. Does the patient appear volume depleted? Try a standard bolus – if this improves his status, you are on the right track.
Now, we look at contractility.
How FAST you FILL the PUMP and SQUEEZE
Is there a problem with the PUMP? That is, with contractility? Is this in an infarction, an infection, a poisoning? Look for signs of cardiac congestion on physical exam. Put the probe on the patient’s chest, and look for effusion. Look to see if there is mild, moderate, or severe decrease in cardiac contractility. If this is cardiogenic shock – a problem with the pump itself -- begin pressors.
And finally, we look to the peripheral vascular resistance.
How FAST you FILL the PUMP and SQUEEZE
Is there a problem with systemic vascular resistance – the SQUEEZE?
Look for signs of changes in temperature – is the patient flushed? Is this an infectious etiology? Are there neurogenic or anaphylactic concerns? After assessing the heart rate, optimizing volume status, evaluating contractility, is the cause of the shock peripheral vasodilation? If so, treat the cause – perhaps this is a distributive problem due to anaphylaxis. Treat with epinephrine. The diagnosis of exclusion in trauma is neurogenic shock. Perhaps this is warm shock, both are supported with norepinephrine. All of these affect systemic vascular resistance – and the shock won’t be reversed until you optimize the peripheral squeeze.
Summary
The four take-home points in the approach to shock in children
- To prioritize your innterventions, remember how patients COHDe: Cardiogenic, Obstructive, Hypovolemic, and Distributive. Your patient's shock may be multifactorial, but mentally prioritize what you think is the MAIN case of the shock, and deal with that first.
- To treat shock, remember: How FAST You FILL The PUMP and SQUEEZE: Look at the heart rate – how FAST. Look at the volume status – the FILL. Assess cardiac contractility – the PUMP, and evaluate the peripheral vascular tone – the SQUEEZE.
- In pediatric sepsis, the most common type is cold shock – use epinephrine (adrenaline) to get that heart to increase the cardiac output. In adolescents and adults, they more often present in warm shock, use norepinephrine (noradrenaline) for its peripheral squeeze to counteract this distributive type of shock.
- Rapid-fire word association:
- Epinephrine for cardiogenic shock
- Intervention for obstructive shock
- Fluids for hypovolemic shock
- Norepinephrine for distributive shock
References
This post and podcast are dedicated to Natalie May, MBChB, MPHe, MCEM, FCEM for her collaborative spirit, expertise, and her super-charged support of #FOAMed. You make a difference. Thank you.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
How do you approach the child who may be altered?
Altered mental status in children can be subtle. Look for age-specific behaviors that range from irritability to anger to sleepiness to decreased interaction.
In the altered child, anchoring bias is your biggest enemy. Keep your mind open to the possibilities, and be ready to change it, when new information becomes available.
For altered adults, use AEIOU TIPS (Alcohol-Epilepsy-Insulin-Overdose-Uremia-Trauma-Infection-Psychosis-Stroke).
Try this for altered children: remember that they need their VITAMINS!
V – Vascular (e.g. arteriovenous malformation, systemic vasculitis)
I – Infection (e.g. meningoencephalitis, overwhelming alternate source of sepsis)
T – Toxins (e.g. environmental, medications, contaminated breast milk)
A – Accident/abuse (e.g. non-accidental trauma, sequelae of previous trauma)
M – Metabolic (e.g. hypoglycemia, DKA, thyroid disorders)
I – Intussusception (e.g. the somnolent variant of intussusception, with lethargy)
N – Neoplasm (e.g. sludge phenomenon, secondary sepsis, hypoglycemia from supply-demand mismatch)
S – Seizure (e.g. seizure and its variable presentation, especially subclinical status epilepticus)
Case One: Sleepy Toddler
16-month-old who chewed on his grandmother's clonidine patch
Clonidine is an alpha-2 agonist with many therapeutic indications including hypertension, alcohol withdrawal, smoking cessation, perimenopausal symptoms. In children specifically, clonidine is prescribed for attention deficit hyperactivity disorder, spasticity due to cerebral palsy and other neurologic disorders, and Tourette’s syndrome.
The classic clonidine toxidrome is altered mental status, miosis, hypotension, bradycardia, and bradypnea. Clonidine is on the infamous list of “one pill can kill”.
Treatment is primarily supportive, with careful serial examinations of the airway, and strict hemodynamic monitoring.
Naloxone can partially counteract the endogenous opioids that are released with clonidine's pharmacodynamics.
Start with the usual naloxone dose of 0.01 mg/kg, up to the typical adult starting dose is 0.4 mg.
In clonidine overdose, however, you may need to increase the naloxone dose (incomplete and variable activity) up to 0.1 mg/kg. Titrate to hemodynamic stability and spontaneous respirations, not full reversal of all CNS effects.
Case Two: In Bed All Day
A 7-year-old with fever, vomiting, body aches, sick contacts. Altered on exam.
Should you get a CT before LP?
If you were going to perform CT regardless, then do it.
Adult guidelines: age over 60, immunocompromised state, history of central nervous system disease, seizure within one week before presentation, abnormal level of consciousness, an inability to answer two consecutive questions correctly or to follow two consecutive commands, gaze palsy, abnormal visual fields, facial palsy, arm drift, leg drift, and abnormal language.
Children: if altered, and your differential diagnosis is broad (especially if you may suspect tumor, bleed, obvious abscess).
Influenza is often overlooked as a potential cause of altered mental status. Many authors report a broad array of neurological manifestations associated with influenza, such as altered mental status, seizures, cranial nerve abnormalities, hallucinations, abnormal behavior, and persistent irritability. All of this is due to a hypercytokinemic state, not a primary CNS infection.
Case Three: Terrible Teenager
14-year-old brought in for "not listening" and "acting crazy"; non-complaint on medications for systemic lupus erythematosus (SLE).
SLE is rare in children under 5. When school-age children present with SLE, they typically have more systemic signs and symptoms. Teenagers present like adults. All young people have a larger disease burden with lupus, since they have many more years to develop complications.
Lupus cerebritis: high-dose corticosteroids, and possibly IV immunoglobulin. Many will need therapeutic plasma exchange (TPE), a type of plasmapheresis.
Summary
- In altered mental status, keep your differential diagnosis open
- Pursue multiple possibilities until you are able to discard them
- Be ready to change your mind completely with new information
- Make sure your altered child gets his VITAMINS (Vascular, Infectious, Toxins, Accident/Abuse, Metabolic, Intussusception, Neoplasm, Stroke)
References
Zorc JJ. A lethargic infant: Ingestion or deception? Pediatr Ann 2000; 29: 104–107
This post and podcast are dedicated to Teresa Chan, HBSc, BEd, MD, MS, FRCPC for her boundless passion for and support of #FOAMed, for her innovation in education, and for her dedication to making you and me better clinicians and educators. Thank you, T-Chan.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
It's a busy shift. Today no one seems to have a chief complaint.
Someone sends a troponin on a child. Good, bad, or ugly, how are you going to interpret the result?
And while we’re at it – what labs do I need to be careful with in children – sometimes the normal ranges of common labs can have our heads spinning!
Read on to go from bread-and-butter pediatric blood work to answer the question – what’s up with troponin, lactate, d-dimer, and BNP in kids?
A fundamental tenet of emergency medicine:
We balance our obligation to detect a dangerous condition with our suspicion of the disease in given patient.
Someone with a cough and fever may simply have a viral illness, or he may have pneumonia. Our obligation is to evaluate for the pneumonia. It’s ok if we “miss” the diagnosis of a cold. It could be bad if we don’t recognize the pneumonia.
How do we decide? Another fundamental concept:
The threshold.
Depending on the disease and the particular patient, we have a threshold for testing, and the threshold for treating. Every presentation – and every patient for that matter – has a complicated interplay between what we are expected to diagnose, how much we suspect that particular serious diagnosis, and where testing and treating come into play.
What's wrong with "throwing on some labs"?
Easy to do right? They are but a click away…
Often a good history and physical exam will help you to calibrate your investigational thresholds. This is especially true in children – the majority of pediatric ambulatory visits do not require blood work to make a decision about acute care. If your patient is ill, then by all means; otherwise, consider digging a bit deeper into the history, get collateral information, and make good use of your general observation skills.
First, a brief word about basic labs.
The punchline is, use a pediatric reference.
If you don’t have a trusted online reference available during your shift, make sure you have something like a Harriett Lane Handbook accessible to you. Don’t rely on your hospital’s lab slip or electronic medical record to save you, unless you are sure that they use age-specific pediatric reference ranges to flag abnormal values. Believe it or not, in this 21st century of ours, some shops still use adult reference ranges when reporting laboratory values on children.
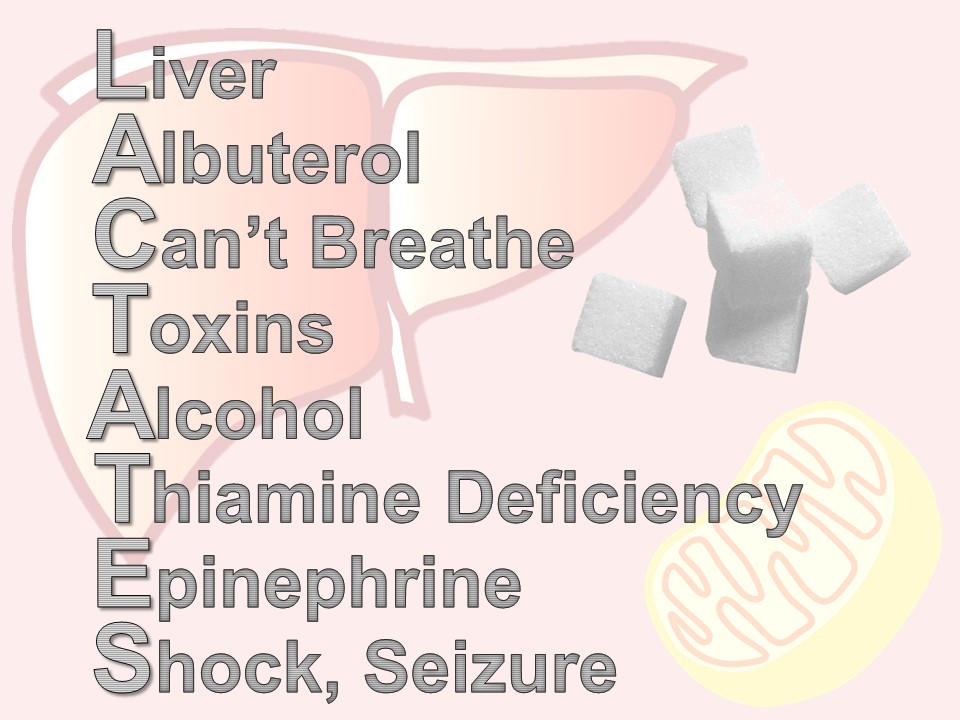
Notable differences in basic chemistries
Potassium: tends to run a bit higher in infants, because for the first year of life, your kidneys are inefficient in excreting potassium.
BUN and creatinine: lower in children due to less muscle mass, and therefore less turnover (and usually lack of other chronic disease)
Glucose: tends to run lower, as children are hypermetabolic and need regular feeding (!)
Alkaline phosphatase: is always high in normal, growing children, due to bone turn over (also fond in liver, placenta, kidneys)
Ammonia: high in infancy, due to immature liver, trends down to normal levels by toddlerhood
ESR and CRP: low in healthy children, as chronic inflamation from comorbidities is not present; both increase steadily with age
Thyroid function tests: all are markedly high in childhood, not as a sign of disease, but a marker of their increased metabolic activity
Big Labs
Troponin
Reliably elevated in myocarditis, and may help to distinguish this from pericarditis (in addition to echocardiography)
Other causes of elevated troponin in children include: strenuous activity, status epilepticus, toxins, sepsis, myocardial infarction (in children with congenital anomalies). Less common causes of troponemia are: Kawasaki disease, pediatric stroke, or neuromuscular disease.
Don't go looking, if you won't do anything with the test.
Brain natriuretic peptide (BNP)
In adults, we typically think of a BNP < 100 pg/mL as not consistent with symptoms caused by volume overload.
Luckily, we have data in children with congenital heart disease as well. Although each company's assay reports slightly different cut-offs, in general healthy pediatric values match healthy adult values.
One exception is in the first week of life, when it is high even in healthy newborns, due to the recent transition from fetal to newborn circulation.
Use of BNP in children has been studied in both clinic and ED settings. Cohen et al. in Pediatrics used BNP to differentiate acute heart failure from respiratory disease in infants admitted for respiratory distress. They compared infants with known CHF, lung disease, and matched them with controls.
Later, Maher et al. used BNP in the emergency department to differentiate heart failure from respiratory causes in infants and children with heart failure and those with no past medical history.
The bottom line is:
BNP reliably distinguishes cardiac from respiratory causes of shortness of breath in children with a known diagnosis of heart failure.
D-dimer
To cut to the chase: d-dimer for use as a rule-out for pulmonary embolism has not been studied in children.
The only data we have in using d-dimer in children is to prognosticate in established cases. It is only helpful to track therapy for children who have chronic clots.
This is where our adult approach can get us into trouble. Basically, think of the d-dimer in children like it doesn’t even exist. It’s not helpful in our setting for our indications. An adult may have an idiopathic PE – in fact, up to a third of adults with PE have no known risk factor, which makes decision tools and risk stratification important in this population.
Children with PE almost always have a reason for it.
There is at least one identifiable risk factor in up to 98% of children with pulmonary embolism. The majority have at least two risk factors.
If you’re suspecting deep venous thrombosis, perform ultrasonography, and skip the d-dimer.
If you’re worried about PE, go directly to imaging. In stable patients, you may elect to use MR angiography or VQ scan, but most of us will go right to CT angiography. Radiation is always a concern, but if you need to know, get the test.
This is yet another reminder that your threshold is going to be different in children when you think about PE – they should have a reason for it. After you have excluded other causes of their symptoms, if they have risk factors, and you are still concerned, then do the test you feel you need to keep this child safe.
You are the test.
Risk factors only inform you, and you’ll have to just pull the trigger on testing in the symptomatic child with risk factors.
Lactate
A sick child with sepsis syndrome?
The short answer – yes.
In the adult literature, we know that a lactate level above 4 mmol/L in patients with severe sepsis was associated with the need for critical care. This has been studied in children as well, and an elevated lactate in children – typically above 4 – was a predictor of prolonged ICU course and mortality in septic patients.
The acute recognition and treatment of sepsis is first and foremost, clinical.
And it’s all about perfusion and providing oxygen to the tissues. Lactate and other laboratory testing is not a substitute for clinical assessment – it should be used as an extension of your assessment. There are two main reasons for an elevated lactate: the stress state and the shock state.
The stress state is due to hypermetabolism and an increase in glycolysis, as an example, in early sepsis. The shock state is due to tissue hypoxia, seen in septic shock. The confusion and frustration with lactate is that we often test the wrong people for it.
We could use it to track treatment, and see if we can clear the lactate; decreased lactate levels are associated with a better outcome in adults. Serial clinical assessments are even more useful to gauge your success with treatment.
We should use lactate to detect occult shock. Children compensate so well for shock, that subtle tissue hypoxia may not be detected until later. It may inform your decision for level of care, intensive care versus some other lower level.
Have you every been in this situation:
"Why, oh why, did we send a lactate?"
There are times when a lactate is ordered – maybe by protocol or maybe accidentally – or maybe in retrospect, the patient didn’t need it. Here is a quick mnemonic to remember the reasons for an elevated lactate: LACTATES
L – liver – any liver disease affects how lactate is metabolized by the Cori cycle
A – albuterol (or for our international friends, salbutamol), beta-agonists like albuterol, increase lactate production via cyclic amp
C – “can’t breathe” – respiratory distress and increased work of breathing shifts the ratio of aerobic and anerobic repiration
T – toxins – all kinds of wonder drugs and recreational drugs do it – look up your patient’s list if you’re suspicious
A – alcohol, not an infrequent offender
T – thiamine deficiency – think of this in your cachectic or malnourished patients
E – epinephrine – a by-product of the cori cycle, how lactate is metabolized. Difficult to interpret lactates when a patient is on an epinephrine drip.
S – seizure or shock – most commonly septic, but can be any type: cardiogenic, bstructive, hypovolemic, distributive.
Bottom line: high serum lactate levels have been associated with morbidity and mortality in children with sepsis and trauma, the two best-studied populations.
A summary of how labs can help you – or hurt you – in pediatric emergency medicine:
- Have a good reference for normal values and always be skeptical of how your lab reports them.
- Troponin testing is great for the child with suspected cardiogenic shock, myocarditis, or in unwell children with congenital heart disease.
- BNP in children can be used just like you do in adults – to get a sense of whether the presenting symptoms are consistent with heart failure.
- D-dimer is mostly a waste of time in the PED.
- Lactate can be useful in the right patient – use it to risk-stratify the major trauma patient or the patient with sepsis that may be suffering from occult shock.
- And lastly, make sure that you are mindful of your threshold for testing, and our threshold for treatment. If will vary by disease and by the patient at hand.
References
Troponin
Gupta SK, Naheed Z. Chest Pain in Two Athletic Male Adolescents Mimicking Myocardial Infarction. Pediatr Emer Care. 2014;30: 493-495.
Kelley WE, Januzzi JL, Christenson RH. Increases of Cardiac Troponin in Conditions other than Acute Coronary Syndrome and Heart Failure. Clinical Chemistry. 2009; (55) 12:2098–2112.
Kobayashi D, Aggarwal S, Kheiwa A, Shah N. Myopericarditis in Children: Elevated Troponin I Level Does Not Predict Outcome. Pediatr Cardiol. 2012; 33:1040–1045.
Koerbin G, Potter JM, Abhayaratna WP et al. The distribution of cardiac troponin I in a population of healthy children: Lessons for adults. Clinica Chimica Acta. 2016; 417: 54–56.
Liesemer K, Casper TC, Korgenski K, Menon SC. Use and Misuse of Serum Troponin Assays in Pediatric Practice. Am J Cardiol. 2012;110:284 –289.
Newby KL et al. for the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF 2012 Expert Consensus Document on Practical Clinical Considerations in the Interpretation of Troponin Elevations. J Am Coll Cardiol. 2012; 60(23): 2427-2463.
Schwartz MC, Wellen S, Rome JJ et al. Chest pain with elevated troponin assay in adolescents. Cardiology in the Young; 2013. 23: 353–360.
BNP
Auerbach SR, Richmond ME, Lamour JM. BNP Levels Predict Outcome in Pediatric Heart Failure Patients Post Hoc Analysis of the Pediatric Carvedilol Trial. Circ Heart Fail. 2010;3:606-611.
Cohen S, Springer C, Avital A et al. Amino-Terminal Pro-Brain-Type Natriuretic Peptide: Heart or Lung Disease in Pediatric Respiratory Distress? Pediatrics. 2005;115:1347–1350.
Fried I, Bar-Oz B, Algur N et al. Comparison of N-terminal Pro-B-Type Natriuretic Peptide Levels in Critically Ill Children With Sepsis Versus Acute Left Ventricular Dysfunction. Pediatrics. 2006; 118(4): 1165-1168.
Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–878.
Maher KO, Reed H, Cuadrado A et al. , B-Type Natriuretic Peptide in the Emergency Diagnosis of Critical Heart Disease in Children. Pediatrics. 2008;121:e1484–e1488.
Mir TS, Marohn S, Laeer S, Eistelt M. Plasma Concentrations of N-Terminal Pro-Brain Natriuretic Peptide in Control Children From the Neonatal to Adolescent Period and in Children With Congestive Heart Failure. Pediatrics. 2002;110(6)1:6.
Mir TS, Laux R, Hellwege HH et al. Plasma Concentrations of Aminoterminal Pro Atrial Natriuretic Peptide and Aminoterminal Pro Brain Natriuretic Peptide in Healthy Neonates: Marked and Rapid Increase After Birth. Pediatrics. 2003;112:896–899.
D-Dimer
Goldenberg NA, Knapp-Clevenger RA, Manco-Johnson MJ. Elevated Plasma Factor VIII and d-Dimer Levels as Predictors of Poor Outcomes of Thrombosis in Children for the Mountain States Regional Thrombophilia Group. Pediatrics. 2003;112:896–899.
Manco-Johnson MJ. How I treat venous thrombosis in children. Blood. 2006; 107(1)21-31.
Naqvi M, Miller P, Feldman L, Shore BJ. Pediatric orthopaedic lower extremity trauma and venous thromboembolism. J Child Orthop. 015;9:381–384.
Parasuraman S, Goldhaber SZ. Venous Thromboembolism in Children. Circulation. 2006;113:e12-e16.
Strouse JJ, Tamma P, Kickler TS et al. D-Dimer for the Diagnosis of Venous Thromboembolism in Children. N Engl J Med. 2004;351:1081-8.
Lactate
Andersen LW, Mackenhauer J, Roberts JC et al. Etiology and therapeutic approach to elevated lactate. Mayo Clin Proc. 2013; 88(10): 1127–1140.
Bai et al. Effectiveness of predicting in-hospital mortality in critically ill children by assessing blood lactate levels at admission. BMC Pediatrics. 2014; 14:83.
Scott HF, Donoghue AJ, Gaieski DF et al. The Utility of Early Lactate Testing in Undifferentiated Pediatric Systemic Inflammatory Response Syndrome. Acad Emerg Med. 2012; 19:1276–1280.
Shah A, Guyette F, Suffoletto B et al. Diagnostic Accuracy of a Single Point-of-Care Prehospital Serum Lactate for Predicting Outcomes in Pediatric Trauma Patients. Pediatr Emer Care. 2013; 29:715-719.
Topjian AA, Clark AE, Casper TC et al. for the Pediatric Emergency Care Applied Research Network. Early Lactate Elevations Following Resuscitation From Pediatric Cardiac Arrest Are Associated With Increased Mortality. Pediatr Crit Care Med. 2013; 14(8): e380–e387.
This post and podcast are dedicated to Daniel Cabrera, MD for his vision and his leadership in thinking 'outside the box'.
Troponin | BNP | D-Dimer | Lactate
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
A 5-year-old boy was playing with his older brother in front of their home when he was struck by a car. He sustained a femur fracture, splenic laceration, and blunt head trauma – the so-called Waddell’s triad.
On arrival, he was in compensated shock, with tachycardia.
He decompensates and needs blood.
How do we manage his hemodynamics and when do we perform massive transfusion?
Pediatric Massive Transfusion
40 mL/kg of blood products given at any time within the first 24 hours.
Adolescents and Adult Massive Transfusion
6-8 units of packed red blood cells (PRBCs)
- Adults have about 5 L of circulating blood.
- Not including plasma, one could replace all circulating erythrocytes with about 10 units of PRBCS
- The best ratio of PRBCs:Plasma:Platelets is unknown, but consensus is 1:1:1.
- 1 unit of PRBCS is typically 300 mL of volume.
The typical initial transfusion of PRBCs in children is 10 mL/kg.
Massive transfusion in children is defined as 40 mL/kg of any blood product.
Once you start to give a child with major trauma the second 10 mL/kg dose of PRBCs – start thinking about other blood components, and ask yourself whether you should initiate your massive transfusion protocol.
The goal is to have the products ready to use in the case of the dynamic trauma patient.
The Thromboelastogram (TEG)
Direct measures the four components of clot formation. When there is endolethial damage and bleeding, the sequence that your body takes to address it is as follows:
- Platelets migrate and form a plug
- Clotting factors aggregate and reinforce the platelets
- Fibrin arrives an acts like glue
- Other cells migrate and support the clot.
R time – reaction time – the initial line in the tracing that shows time to beginning of clot formation.
- Treated with platelets
K factor – kinetics of the clot –how much the clot allows the pin to move, or the amplitude.
- Treated with cryoprecipitate
Alpha angle – the slope between the R and K measurements – reflects how quickly the fibrin glue is working.
- Treated with cryoprecipitate
Ma – maximum amplitude – reflects the overall strength of the clot.
- Treated with platelets
LY30 – the clot lysis at 30 min – is the decrease in strength of the clot’s amplitude at 30 min.
- Treated with an antifibrinolytics (tranexamic acid)
Shape Recognition
Red wine glass: a normal tracing with a normal reaction time and a normal amplitude. That patient just needs support and monitoring.
Champagne glass: a coagulopathic TEG tracing – thinned out, with less amplitude. This patient needs specific blood products.
Puffer fish or blob: a hyperfibrinolytic tracing. That patient will needs clot-stablizer.
TEG – like the FAST – can be repeated as the clinical picture changes.
The Trauma Death Spiral
Lethal triad of hypothermia, acidosis, and coagulopathy.
Keep the patient perfused and warm.
Each unit of PRBCs contains 3 g citrate, which binds ionized calcium, causing hypotension. In massive transfusion, give 20 mg/kg of calcium chloride, up to 2 g, over 15 minutes. Calcium chloride is preferred, as it is ionically readily available – just use a larger-bore IV and watch for infiltration. Calcium gluconate could be used, but it requires metabolism into a bioavailable source of calcium.
Prothrombin complex concentrate (PCC)
Prothrombin complex concentrate (PCC) is derived from pooled human plasma and contains 25-30 times the concentration of clotting factors as FFP. Four-factor PCCs contain factors II, VII, IX and X, while 3-factor PCCs contain little or no factor VII.
The typical dose of PCC is 20-50 units/kg
In the severely hemorrhaging patient – you don’t have time to wait for the other blood products to thaw – PCC is a powder that is reconstituted instantly at the bedside.
Tranexamic acid (TXA)
Tranexamic acid (TXA), is an anti-fibrinolytic agent that functions by stopping the activation of plasminogen to plasmin, and the degradation of fibrin. The Clinical Randomisation of an Antifibrinolytic in Significant Hemorrhage (CRASH-2) investigators revealed a significant decrease in death secondary to bleeding when TXA was administered early following trauma.
Based on the adult literature, one guideline is to give 15 mg/kg loading dose of TXA with a max 1 g over 10 minutes followed by 2 mg/kg/h for at least 8 h or until bleeding stops.
Resuscitative Pearls
Our goal here is damage control. Apply pressure whenever possible. Otherwise, resuscitate, identify the bleeding source, and slow or stop the bleeding with blood products or surgery.
How Children are Different in Trauma
In adults, we speak of “permissive hypotension” (also called “balanced resuscitation” or “damage control resuscitation”). The idea is that if we bring the adult patient’s blood pressure up to normal, we may be promoting clot rupture. To avoid this, we target a MAP of 65 and look for clinical signs of sufficient perfusion. Adults tolerate hypotension relatively well, and is sufficient until we send them to the OR or interventional radiology suite.
In children, this is simply not the case. Hypotension in children is a sign of pre-arrest. Remember, they compensate with an increased systemic vascular resistance and tachycardia to maintain blood pressure.
We should not allow children to become hypotensive – severe tachycardia alone should prompt us to resuscitate.
In other words, permissive hypotension is not permissible for children.
FAST is not sensitive enough to rule-out abdominal trauma.
Fox et al in Academic Emergency Medicine found a sensitivity of 52%; with a 95% confidence interval [CI] = 31% to 73%.
Often children even with high-grade splenic and liver lacerations can be managed non-operatively. If they are supported adequately, they are observed in the ICU and can avoid surgery in many cases. Unfortunately, a negative FAST cannot help with detecting or grading the laceration for non-operative management. In other words, feel free to use ultrasound – especially for things that we in the ED will react to and intervene on – but CT may help to manage the traumatized child non-operatively.
General Guideline for Imaging in Pediatric Trauma
CT Head and Neck, non-contrast: in concerning mechanisms of injury, patients that are difficult to assess (especially those under 3 months), those with a GCS of 13 or lower.
CT Chest, IV contrast: for suspicion of vascular injury that needs exploration, especially in penetrating trauma. Otherwise, chest xray will tell you everything you need to know in children – especially in blunt trauma. Hemo or pneumothoraces are readily picked up by US or CXR. Rib fractures on CXR predict pulmonary contusions. If you are concerned about great vessel injury, then CT Chest may be helpful; otherwise consider omitting it.
CT Abdomen and Pelvis, IV contrast: helpful in grading splenic and liver lacerations with goal to manage non-operatively. Abdominal tenderness to palpation, significant bruising, or a seat belt sign are concerning and would generally warrant a CT. Also, consider in liver function test abnormalities, or hematuria.
Extremity injuries: in general can be evaluated with physical exam and plain films. However, some injuries in high-risk anatomically complex areas such as the hand and wrist, tibial plateau, and midfoot may be missed by plain films, and CT may be helpful here.
Remember: you can help to mitigate post-traumatic stress and risk for adult healthcare aversion.
Summary
- Massive transfusion in children is at 40 mL/kg of total blood products. Think about it if you are giving your second transfusion to the traumatized child.
- Do everything you can to support perfusion and avoid the death spiral of hypothermia, coagulopathy, and acidosis. Keep the child perfused with blood as needed, correct coagulopathy, avoid too much crystalloid, and make sure to use the least high-tech of all of these interventions – keep him dry and covered with warm blankets.
- Do a careful physical exam, and use CT selectively with an end-point in mind – the default is not the pan-scan – evaluate possible injuries depending on your suspicions from history, physical, and lab tests.
- Become familiar with the relatively new modalities in trauma such as TXA, cryoprecipitate and the emerging technology of thromboelestogram – red wine is good for you, champagne is weak, and a puffer fish is trouble.
Selected References
This post and podcast are dedicated to Larry Mellick, MS, MD, FAAP, FACEP. Thank you for your dedication to medical education, and sharing your warm bedside manner, extensive knowledge and talents, and your patient interactions with the world.
Powered by #FOAMed — Tim Horeczko, MD, MSCR, FACEP, FAAP
Traumatized children need your full attention.
Protocols work well for adults, but trauma in children requires that we exercise our clinical muscles just a bit more.
Two main reasons:
-
Children have specific injury patterns
-
Their physiologic response to trauma is unique.
Crash course in pediatric anatomy and physiology in trauma
When you think of trauma in children, think of Charlie Brown. Large head, no neck, his chest and abdomen form an underdeveloped, amorphous shape.
Alternatively, think of children as apples – they are rounder than they are tall, with a large increased surface area. Apples don’t have a hard shell or thick rind to protect them. If you drop them, you may not see any evidence of damage to the outside, but there can be considerable bruising just under the surface.
- A child has thin skin, less subcutaneous deposits than an adult, and a non-calcified, pliable thorax that deforms more than it protects or shields.
- The child’s abdominal muscles are not yet developed. There is less peritoneal fat to cushion a blow, and so traumatic forces transmit readily into internal organs, often without external bruising.
- The child’s large surface area also causes him to dissipate heat more quickly. He may be wet from urine or blood, and in a major trauma, this faster cool-down predisposes him to coagulopathy.
Case
A 5-year-old boy who was playing with his older brother in front of their home when the ball rolled into the street. He ran after it, and was struck by a sedan going approximately 30 mph.
This is the so-called Wadell’s triad that occurs in a collision of auto versus pedestrian or auto versus bicycle. The initial impact is the greatest, and will vary depending on the child’s height and what part of his body reaches up to the bumper of the car. Depending on the height of the child and the height of the car, the initial impact will cause a femur fracture, a pelvic fracture, or direct abdominal trauma. The second impact happens as the child is flung onto the grill or the hood of the car, causing usually thoracic trauma. The third impact can be the coup de grace – to add insult to major injury, the child is then propelled forward, worsening the two previous impacts’ injuries and adding a third – severe blunt head trauma.
Intubation Pearl #1:
If your patient has any subtle change in mental status, intubate early. In pediatric trauma, we need to be proactive. Hypoxia is our enemy.
Intubation Pearl #2:
Thankfully cervical spine injuries in children are uncommon, and when they do occur, they typically occur at the child’s fulcrum, which is at C2. Compare this with an adult’s injury pattern with our fulcrum at C7. Be careful and minimize manipulation of the cervical spine, but do what you must to visualize the chords and place the tube. Keep the neck midline, and realize that the child’s usual decrease respiratory reserve is even more affected by trauma. Preoxygenate and pass that tube quickly.
Chest Tube Pearl #1:
Chest tube sizing in pediatrics is straightforward if we remember that the traditional chest tube size is 4 x the ETT size.
Chest Tube Pearl #2:
Try using a pigtail catheter.
Safety Triangle
- Lateral edge of the pectoral muscle
- Lateral edge of the latisimus dorsi
- Line along the fifth intercostal space at the level of the nipple.
It’s roughly where you would put on a generous dose of deodorant. Insertion here minimizes the risk of damage to nerves, vessels and organs.
Resuscitative Thoracotomy in Children
In a 40-year review of ED thoracotomy, Moore et al. analyzed 1,691 patients who received ED thoracotomy. Overall all-cause adult survival was 6.1%. In children ? 15 years of age, overall all-cause survival was considerably less, at 3.4%.
In a large case series and review of the literature for pediatric ED thoracotomy, Allen et al. found a survival rate in penetrating trauma of 10.2%, with a much lower survival rate in blunt pediatric arrest, at 1.6%. Adolescents had more penetrating injuries, and younger children had more blunt trauma.
To synthesize, the rarity of ED thoracotomy in children is due to the fact that:
- Traumatic full arrest in children is uncommon.
- It is most often blunt trauma.
- Blunt traumatic arrest in children is mostly non-survivable.
REBOA
If you have access to resuscitative endovascular balloon occlusion of the aorta or REBOA, this may be an option to temporize the child to get him to the relative control of the operating room. REBOA involves accessing the common femoral artery, passing a vascular sheath, floating a balloon catheter to the appropriate section of the aorta, and inflating the balloon to occlude blood flow.
Brenner et al. described a case series of 6 patients from two Level I trauma centers. They used REBOA for refractory hemorrhagic shock due to either blunt or penetrating injury. After balloon occlusion, blood pressure improved sufficiently to take the patient either to interventional radiology or to the OR. Four patients lived, two died. The AORTA trial is underway to investigate its use in trauma.
Summary:
- Children are like Charlie Brown – large head, no neck, amorphous, underdeveloped and unprotected thorax and abdomen. Or, if you like, they’re like, apples – they have a large surface area and are easily internally bruised, often without overt signs of external bruising.
- Chest tubes for children are very similar to the adult procedure – the traditional chest tube size is 4 x the child’s ETT size. Try to use smaller pigtail catheters, available in commercial kits, whenever possible. They’re easy, safe, and effective.
- Resuscitative thoracotomy is for penetrating trauma with signs of life wthin 10-15 minutes of arrival. Find the correctable surgical cause of the arrest. Resuscitative thoracotomy for blunt trauma has a dismal prognosis in children.
Selected References
Pediatric Trauma on WikEM
This post and podcast are dedicated to Dr Al Sacchetti, MD, FACEP. Thank you for promoting the emergency care of children and for spreading the message that you don’t need subspecialty training to take good care of acutely ill and injured children.
Powered by #FOAMed — Tim Horeczko, MD, MSCR, FACEP, FAAP
In the young child, vomiting is the great imitator:
Gastrointestinal, Neurologic, Metabolic, Respiratory, Renal, Infectious, Endocrine, Toxin-related, even Behavioral.
To help us organize, below is a review of can't-miss diagnoses by age.
The Neonate: Malrotation with Volvulus
In children with malrotation, 50% present within the first month of life, with the majority occurring in the first week after birth. 90% of children with malrotation with volvulus will present by one year of age. This is a pre-verbal child’s disease – which makes it even more of a challenge to recognize quickly.
The sequence of events usually is fussiness, irritability, and forceful vomiting. The vomit quickly turns bilious.
Green vomit is a surgical emergency.
Babies may also present unwell, with bloating and abdominal tenderness to palpation. Be aware that later stages of malrotation may present as shock – they present in hypovolemic shock due to third-spacing from necrotic bowel and/or septic shock from translocation or perforation. In the undifferentiated sick neonate, always consider a surgical emergency such as malrotation with volvulus.
In the stable patient, get an upper GI contrast study.
Rapid-fire word association for other vomiting emergencies in a neonate:
- Fever, irritability and vomiting? Think meningitis, UTI, or sepsis.
- Premature, unwell, and vomiting? Think necrotizing enterocolitis. Remember, 10% of cases of NEC can be full-term. Look for pneumatosis intestinalis.
- Systemically ill, afebrile, and vomiting for no other reason? Think inborn error of metabolism. Screen with a glucose, ammonia, lactate, and urine ketones.
- Others include congenital intestinal atresia or webs, meconium ileus, or severe GERD
The Infant: Non-Accidental Trauma
All that vomits is not necessarily from the gut.
Abusive head injury is the most common cause of death from child abuse. Infants especially present with non-specific complaints like fussiness or vomiting. Up to 30% of infants with abusive head injury may be misdiagnosed on initial presentation.
Louwers et al. in Child Abuse and Neglect developed and validated a six-question screening tool for use the in ED. The power of this tool was that it was validated for any chief complaint – it is not an injury evaluation checklist – it is a screen for potential abuse in the undifferentiated child:
- Is the history consistent?
- Was seeking medical help unnecessarily delayed?
- Does the onset of injury fit with the developmental level of the child?
- Is the behavior of the child and his interaction with his care-givers appropriate?
- Do the findings of the head-to-toe examination match the history?
- Are there any other red flags or signals that make you doubt the safety of the child or other family members?
On multivariable analysis, if at least one of the questions was positive, there was an OR of 189 for abuse (CI 97 – 300). In other words, if any of those six questions are problematic, get your child protective team involved.
Other important diagnoses in the infant: intussusception and pyloric stenosis (rapid review in audio).
The Toddler: Diabetic Ketoacidosis (DKA)
The important diagnosis not to miss in the vomiting toddler or early school age child is the initial presentation of diabetic ketoacidosis. Children under 5 (especially those under 2) and those from lower socioeconomic groups have a higher risk of DKA as their initial presentation of diabetes.
This is true for any child that isn’t quite acting right – check a finger stick blood sugar as a screen.
The International Society for Pediatric and Adolescent Diabetes (ISPAD) criteria for DKA:
- Hyperglycemia, with a blood glucose of >200 mg/dL (11 mmol/L)
AND - Evidence of metabolic acidosis, with a venous pH of less than 7.3 or a bicarbonate level of < 15 mEq/L
AND - Ketosis, found either in the urine or if directly checked in the blood.
If you have access to checking a serum beta-hydroxybutryrate – the unsung ketone – it can help in diagnosis in unclear cases.
Cerebral Edema Criteria:
- Minor criteria: headache, vomiting, irritability or lethargy; hypertension in the face of hypovolemia.
- Major criteria: change in mental status, including agitation or delirium; incontinence (especially if inappropriate for the child’s age); sluggish pupils and cranial nerve palsies; relative bradycardia (Cushing’s triad).
Cerebral Edema Action Items:
- Immediately give mannitol, 1 g/kg over 15-20 minutes. May repeat it in 2 hours if needed. Hypertonic saline (3% NaCl) is second-line therapy.
- Put the head of the bed up 30 degrees.
- Alert your colleagues and counsel your parents. Make sure everyone knows what to watch out for.
As you can see, vomiting in the young child can be really anything! Keep your differential broad, and think by age and by system.
Differential Diagnosis of Vomiting in Children
The general approach to the child with chiefly vomiting starts with the decision: sick or not sick. If ill appearing, establish rapid IV access, or if needed IO. Rapid blood sugar and if available a point of care pH and electrolytes. Be the detective in your history and doggedly go after any red flags as you go methodically by organ system.
- Do a careful physical exam. The general assessment is always helpful – is the child irritable, listless, agitated?
- What is his work of breathing? Effortless tachypnea may be a sign of acidosis or sepsis.
- Is the abdomen soft or is it tender or distended. Always look in the diaper area – is there a hernia, is there a high-riding, tender, discolored scrotum without cremasteric reflex? Ovarian torsion has been reported in infants as young as 7 months.
- Any skin signs? Look for petechiae, urticaria, purpura.
In other words, use your best judgement, have the dangerous differentials in the back of your mind, and pull the trigger when red flags mount up. Otherwise, a good history and a good exam will get you where you need to be.
Take home points for the young child with vomiting:
- Neonates are allowed to regurgitate (effortless reflux of stomach contents -- the happy spitter-upper). They are not allowed to vomit (forceful, unpleasant contraction of abdominal muscles). Consider surgical causes of forceful vomiting, especially if the child does not look anything other than well.
- Bilious is bad – green vomit is always a surgical emergency – do not pass go – get the surgeons involved early
- Not all vomiting is GI related – if it is not obviously benign, think methodically by organ system and adjust your targeted history and physical to pick up any leads.
- Match the tempo of your treatment to the tempo of the disease.
References
Applegate KE, Anderson JM, Klatte EC. Intestinal malrotation in children: a problem-solving approach to the upper gastrointestinal series. Radiographics. 2006; 26(5):1485-500.
Glaser NS, Wootton-Gorges SL, Buonocore MH et al. Frequency of sub-clinical cerebral edema in children with diabetic ketoacidosis. Pediatr Diabetes. 2006 Apr;7(2):75-80.
Louwers ECFM, Korfage IJ, Affourtit MJ et al. Accuracy of a screening instrument to identify potential child abuse in emergency departments. Child Abuse & Neglect. 2014; (38): 1275–1281.
Lee HC, Pickard SS, Sridhar S et al. Intestinal Malrotation and Catastrophic Volvulus in Infancy. J Emerg Med. 2012; 43(1): e49–e51.
Marcin JP, Glaser N, Barnett P et al. Factors associated with adverse outcomes in children with diabetic ketoacidosis-related cerebral edema. J Pediatr. 2002; 141(6):793-7.
Parashette KR, Croffie J. Intestinal Malrotation in Children: A Problem-solving Approach to the Upper Gastrointestinal. Pediatrics in Review. 2013; (34)7: 307-321.
Wolfsdorf JI, Allgrove J, Craig ME et al. ISPAD Clinical Practice Consensus Guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014 Sep;15 Suppl 20:154-79.
This post and podcast are dedicated to Damian Roland, BMedSci (Hons), MB BS, MRCPCH, for his fervor in the care of children and his dedication to quality medical education.
Nausea and Vomiting | Non-Accidental Trauma | DKA
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP