Categories
Dogma often dictates routine care.
There are times when we have to attend to paradigm shifts.
An easy way to save lives? Just say no to (these) drugs:
Codeine
Normally metabolized into codeine-6-glucuronide (50-70%) and norcodeine (10-15%). Codeine, codeine-6-glucuronide, and norcodeine have low affinity for the μ (mu) receptor.
However, the most active metabolite of codeine is morphine with 200x the affinity for the mu receptor as the codeine derivates. The problem is, people vary in its metabolism from 0-15% of codeine is metabolized to morphine.
Ok, codeine is lame at best, unpredictable at worst.
True. Unless you are hiding a genetic time bomb.
You're an ultra-rapid metabolizer.
Some people have multiple extra copies of the DNA sequence for the CYP2D6 enzyme. Ultra rapid metabolizers funnel a huge proportion of their codeine into morphine metabolism, resulting in a bolus of morphine, ending in apnea.
Promethazine with codeine
This combination is no better than placebo -- all of the risks, with no proven benefit. This combination is notoriously abused -- as purple drank or sizzurp. The rapper Pimp C died of this.
Speaking of cough syrups...
The AAP recommends no cough and cold preparations in children under age 6. They have not been adequately studied in young children, and are not recommended for treating the common cold.
What then? You gotta give me something, doctor!
Ok, Honey!
In a study in the Archives of Pediatric and Adolescent Medicine, Dr Paul and colleagues published: Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. They compared a buckwheat honey, honey-flavored dextromethorphan (DM) and no treatment 30 min before bed for children with upper respiratory tract infections.
Of the three, honey, dextromethorphan, and no treatment – honey scored the best for symptom improvement and cough frequency.
Over age 1? Cough and cold? Honey. There is no concern about accidental overdose, parents are doing something with a proven effect, and compliance is pretty much 100% -- and Grandma approves.
Dextromethorphan
No proven benefit over placebo. Also widely abused, in pill form ("Skittles") and/or liquid form mixed in alcoholic beverage ("robotripping").
Alternatives to Codeine
Details in Audio:
Morphine liquid
Acetaminophen and Hydrocodone
PEARLS AND PITFALLS IN PEDIATRIC PAIN
Allow the child to speak for himself whenever possible. After acknowledging the parent’s input, perhaps try “I want to make sure I understand how the pain is for you. Tell me more.”
Engage parents and communicate the plan to them. Elicit their expectations, and give them of preview of what to expect in the ED.
Opioids are meant for pain caused by acute tissue injury, for the briefest period of time feasible. Older school-aged children and adolescents are increasingly at risk for opioid dependence and addiction.
Give detailed advice on how to manage pain at home. Set expectations. Let them know you understand and will help them through your good advice that will carry them through this difficult time. Patients and families often just need a plan. Map it out clearly.
And...
Just say no to: codeine, promethazine with codeine, and dextramethorphan.
Selected References
FDA. Most Young Children With a Cough or Cold Don't Need Medicine.
Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923.
This post and podcast are dedicated to Bryan Hayes, PharmD for his practical approach to pharmacologic conundrums and to David Juurlink, MD, PhD for his steadfast dedication to patient safety and clinician education. Check out Bryan's helpful blog and clinical resource, PharmERToxGuy. Check out David anywhere one utters the word Tra-ma-dol.
Not all head trauma is minor.
Not all minor head trauma is clinically significant.
How can we sort out the overtly ok from the sneakily serious?
Mnemonics for bedside risk stratification of minor pediatric blunt head trauma, based on PECARN studies:
[Details in Audio]
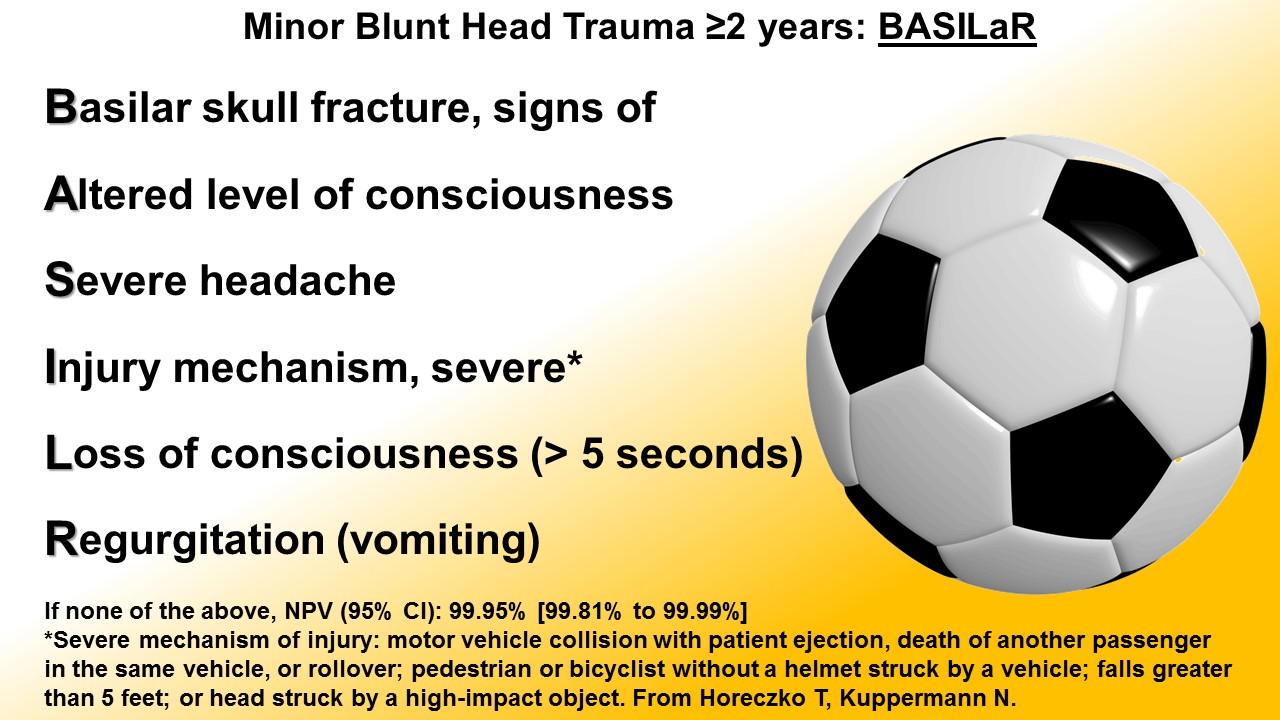
Blunt Head Trauma in Children < 2 years of Age
Blunt Head Trauma in Children ≥ 2 years of Age
Image Gently Campaign
Medical Imaging Record (maintain like an immunization card)
Brochure for Parents: Just in Time Education
Selected References
This post and podcast are dedicated to Kevin Klauer, DO, EJD, FACEP for his dedication to education, and for his unique balance of safety and keeping it real. Thank you.
Comfortable with G-tubes, tracheostomies, and VP shunts?
Good.
Get ready for the next level: Vagus Nerve Stimulators, Intrathecal Pumps, and Ventricular Assist Devices.
Details in Audio:
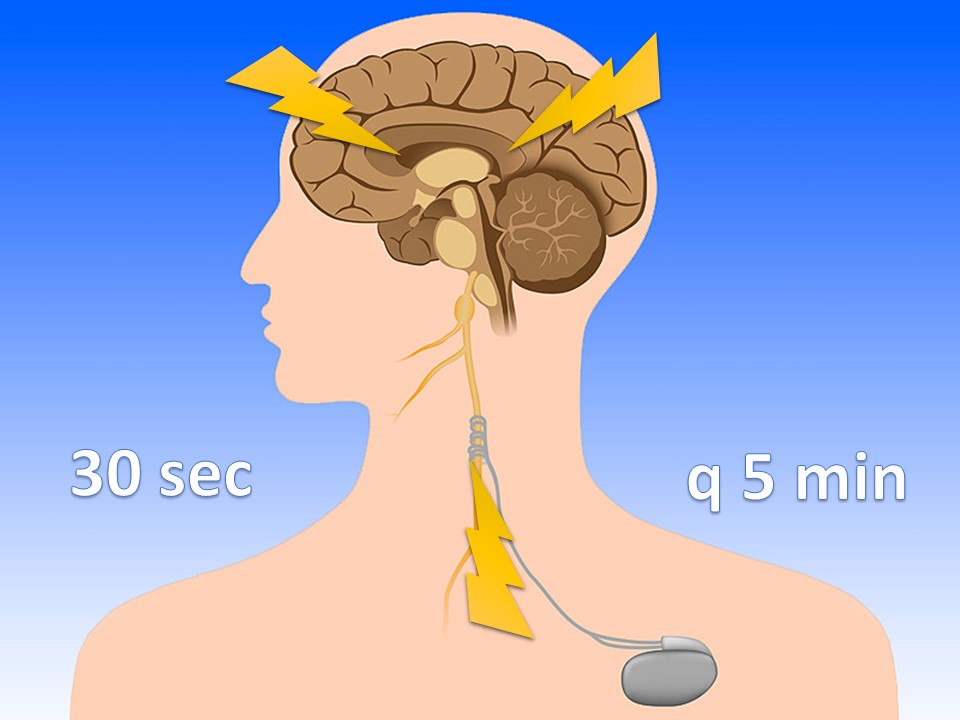
Vagus Nerve Stimulators
For intractable epilepsy; sends retrograde signal up corona radiata
Also may be used in: depression, bulimia, Alzheimer, narcolepsy, addiction, and others
VNS magnets
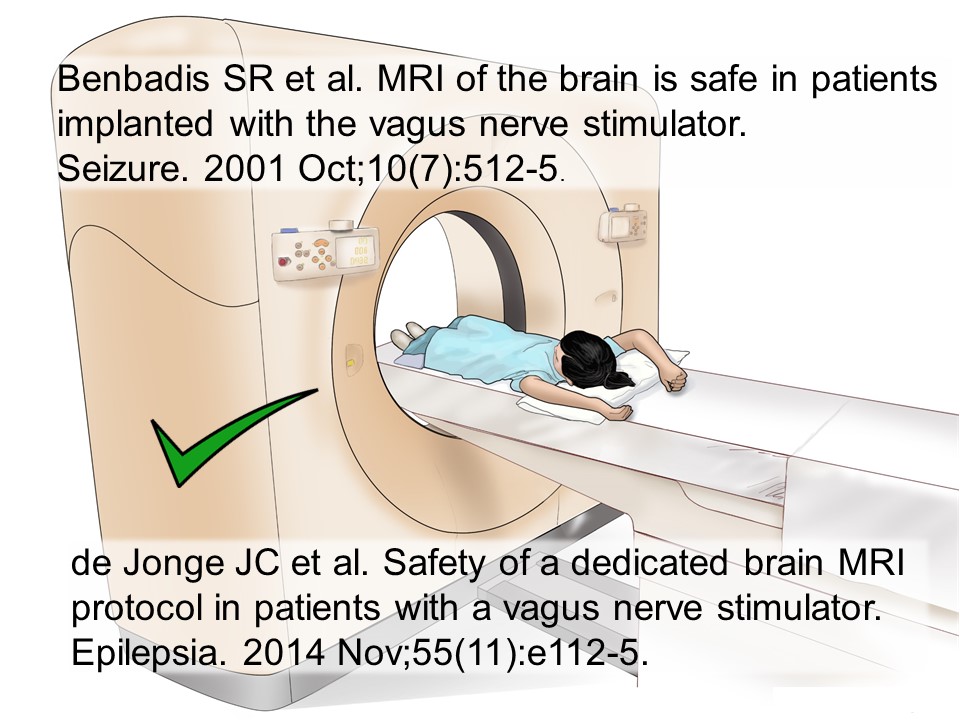
Are VNS safe in MRI?
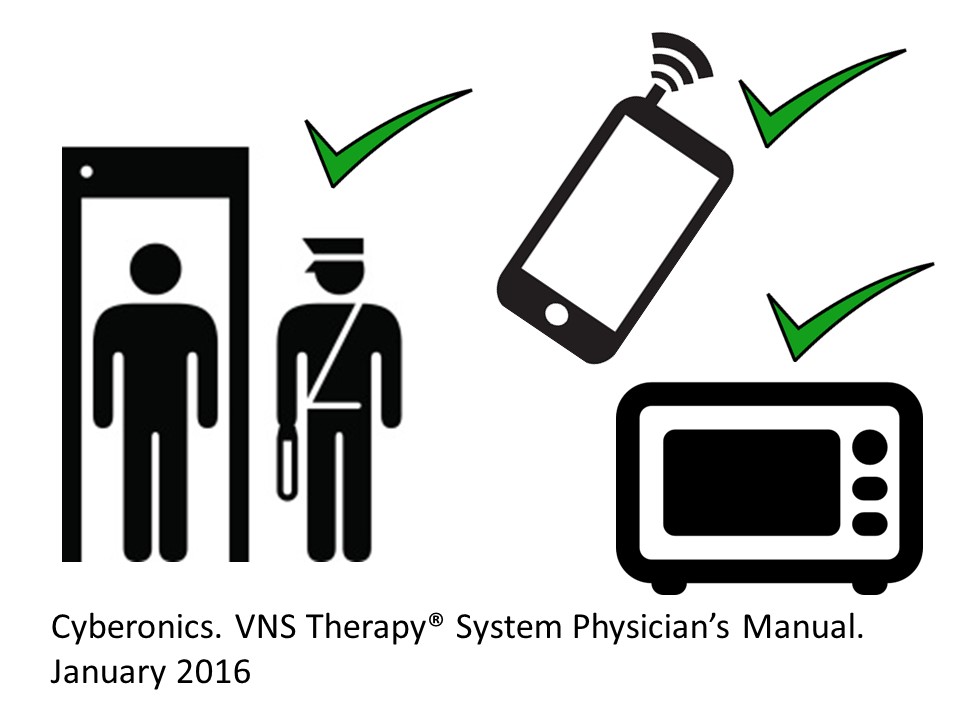
Are VNS safe in everyday life?
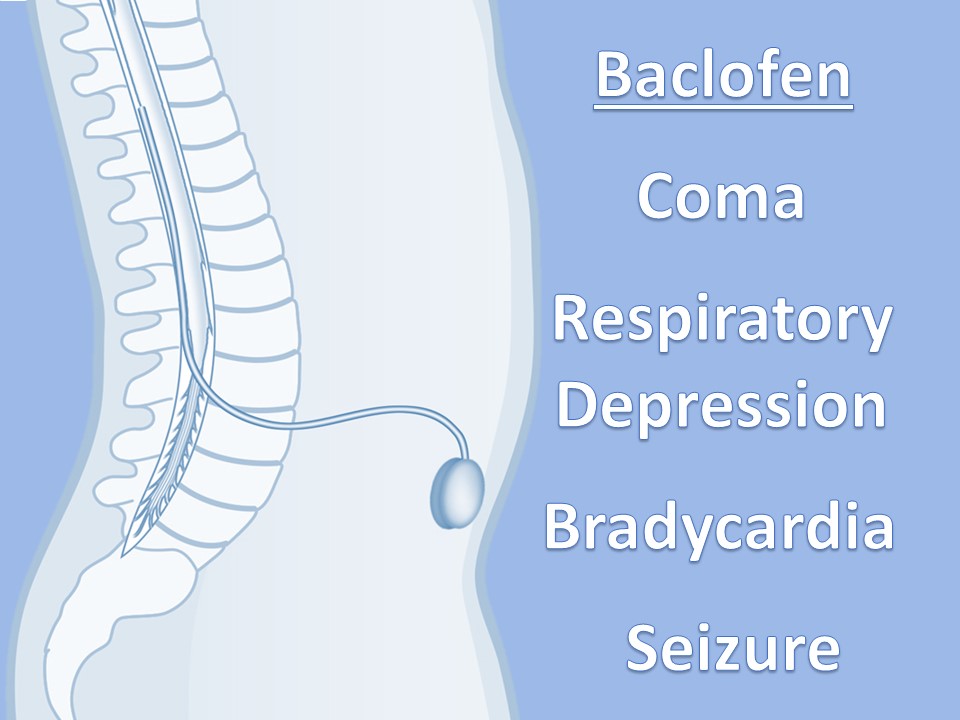
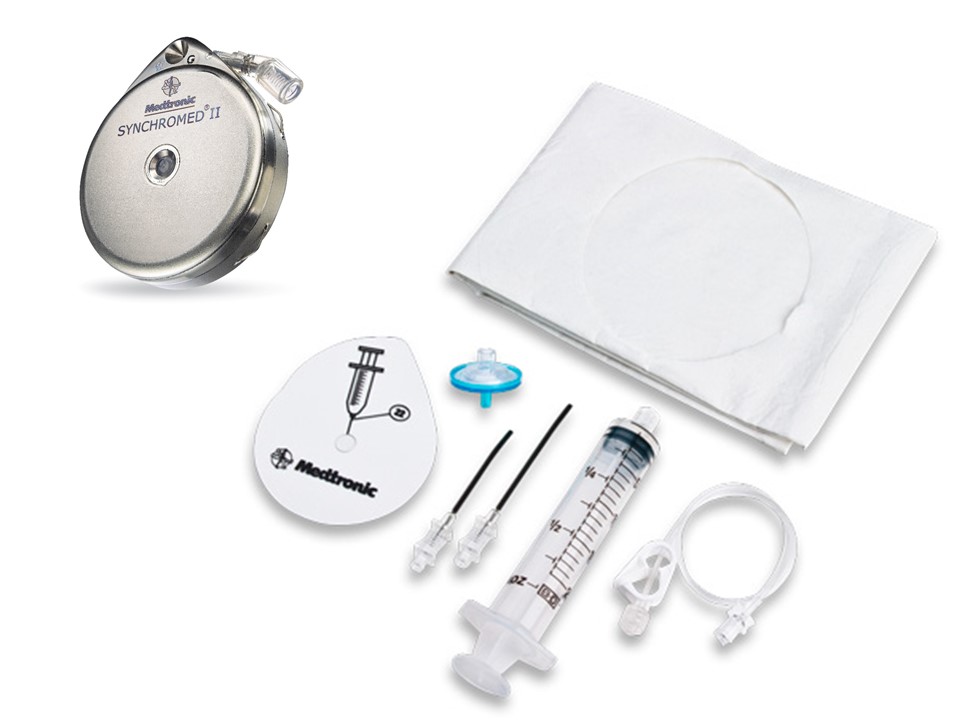
Intrathecal Pumps
Used to infuse basal rate of drug, usually baclofen for spasticity, but pump may contain morphine, bupivicaine, clonidine. Also used for severe MS, stroke, TBI, chronic pain. Verify the medication and identify the toxidrome if symptomatic.
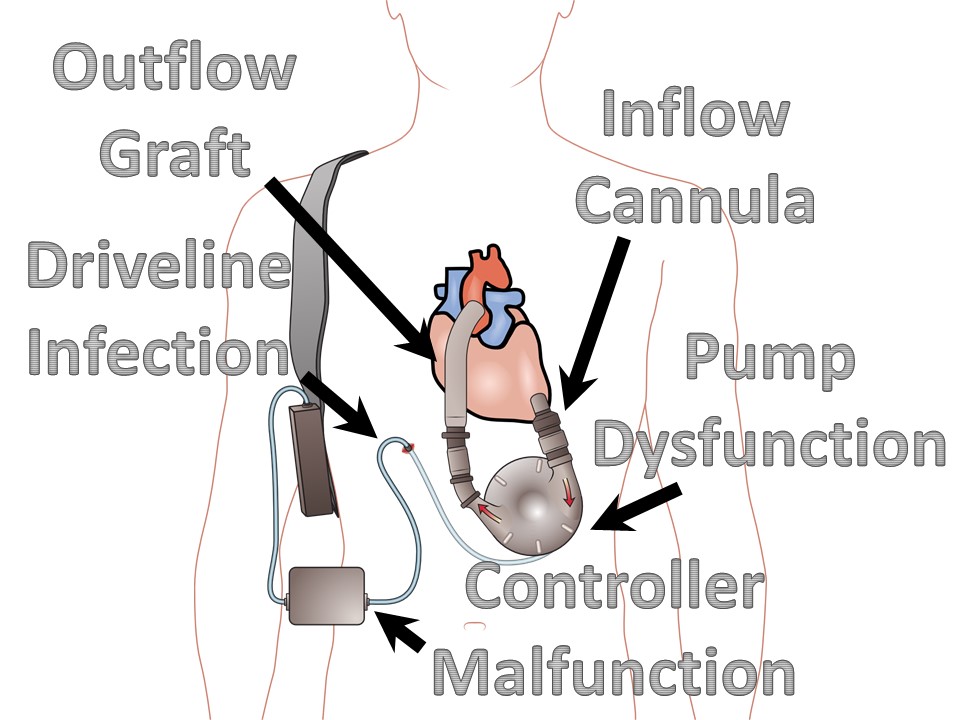
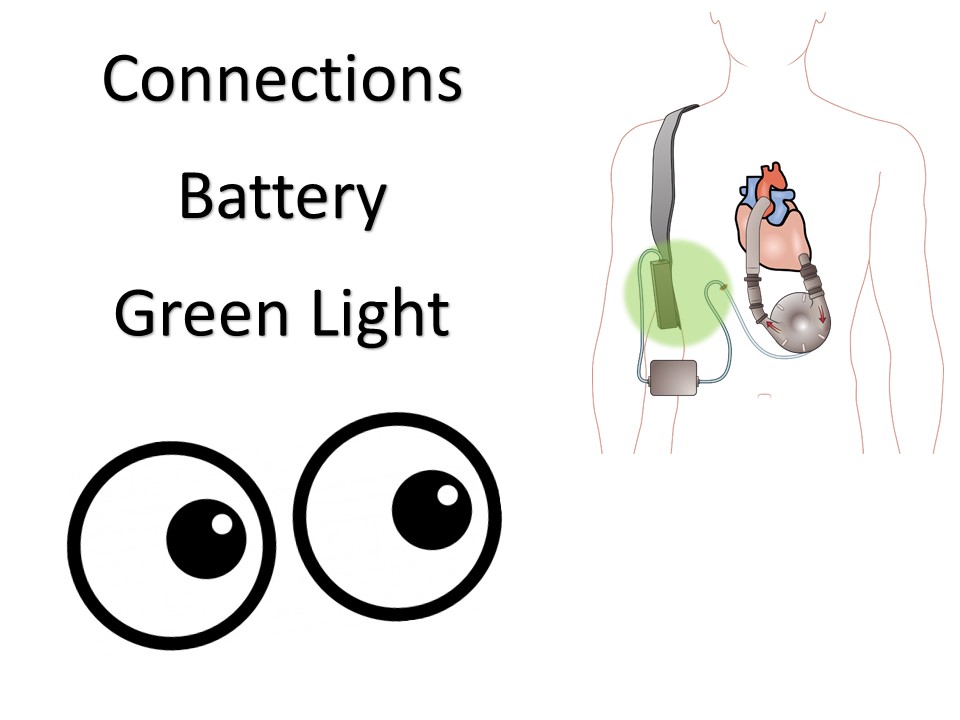
Ventricular Assist Devices
May be left ventricular assist, right ventricular assist, or biventricular assist device.
References
Vagus Nerve Stimulators (VNS)
Elliott RE, Rodgers SD, Bassani L et al. Vagus nerve stimulation for children with treatment-resistant epilepsy: a consecutive series of 141 cases. J Neurosurg Pediatrics. 2011; 7:491-500.
Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neuroscience and Biobehavioral Reviews. 2005; 29: 493–500.
Panebianco M, Rigby A,Weston J,Marson AG. Vagus nerve stimulation for partial seizures. Cochrane Database of Systematic Reviews. 2015; 4, Art. No.: CD002896.
Ruffoli R, Giorgi FS, Pizzanelli C et al. The chemical neuroanatomy of vagus nerve stimulation. Journal of Chemical Neuroanatomy; 2011; 42: 288–296.
Intrathecal Pumps
Borowski A, Littleton AG, Borkhuu B et al. Complications of Intrathecal Baclofen Pump Therapy in Pediatric Patients. J Pediatr Orthop. 2010; 30:76–81.
Ghosh D, Mainali G, Khera J, Luciano M. Complications of Intrathecal Baclofen Pumps in Children: Experience from a Tertiary Care Center. Pediatr Neurosurg. 2013; 49:138–144.
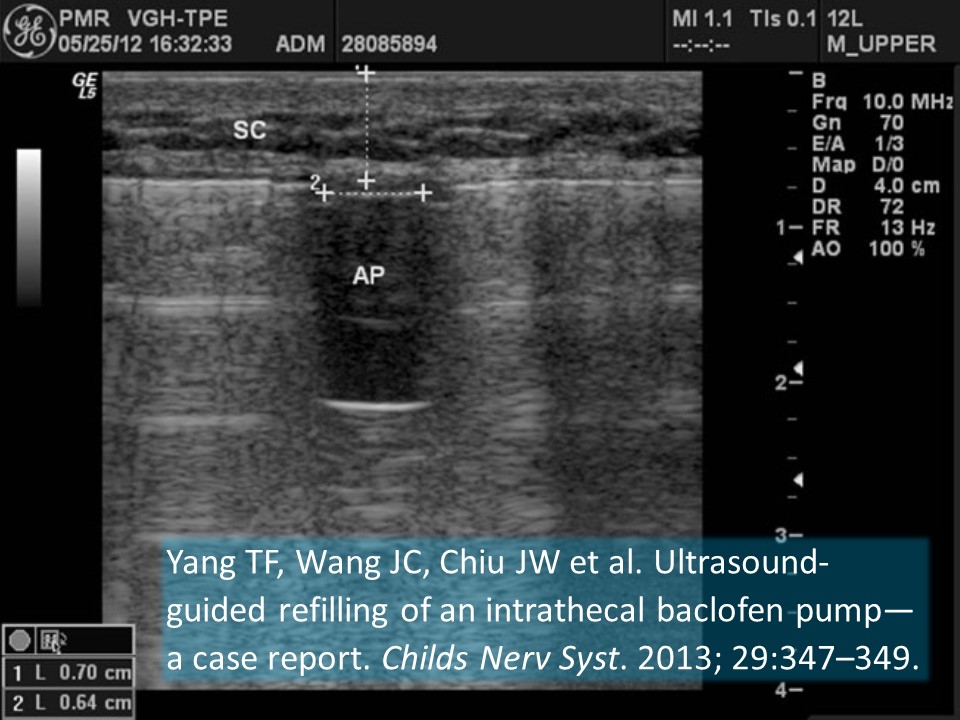
Yang TF, Wang JC, Chiu JW et al. Ultrasound-guided refilling of an intrathecal baclofen pump—a case report. Childs Nerv Syst. 2013; 29:347–349.
Yeh RN, Nypaver MM, Deegan TJ, Ayyangar R. Baclofen Toxicity in an 8-year-old with an Intrathecal Baclofen Pump. J Emerg Med. 2004; 26(4): 163–167.
Ventricular Assist Devices
Blume ED, Naftel DC, Bastardi HJ et al. for the Pediatric Heart Transplant Study Investigators. Outcomes of Children Bridged to Heart Transplantation With Ventricular Assist Devices: A Multi-Institutional Study. Circulation. 2006; 113: 2313-2319.
Colón JE, Laborde ME, Nossaman BD. Case Report: Left Ventricular Assist Device in a 12 Year Old Child as a Bridge to Heart Transplantation. Section of Congenital Cardiac Anesthesia, Ochsner Medical Center, New Orleans, Louisiana. 2012.
Fan Y, Weng YG, Huebler M et al. Predictors of In-Hospital Mortality in Children After Long-Term Ventricular Assist Device Insertion. J Amer Coll Cardiol. 2011; 58(11):1183–90
Fraser CD, Jaquiss RDB, Rosenthal DN et al. Prospective Trial of a Pediatric Ventricular Assist Device. N Engl J Med. 2012;367:532-41.
Gazit AZ, Gandhi SK, Canter CC. Mechanical Circulatory Support of the Critically Ill Child Awaiting Heart Transplantation. Current Cardiology Reviews. 2010; 6: 46-53.
VanderPluym CJ, Fynn-Thompson F, Blume ED. Ventricular Assist Devices in Children Progress With an Orphan Device Application. Circulation. 2014;129:1530-1537.
This post and podcast are dedicated to Joe Bellezzo, MD, FACEP and Zack Shinar, MD, FACEP for bringing us all up to speed. Listen to their fantastic ED ECMO podcast here.
Abdominal pain is common; so are strongly held myths and legends about what is concerning, and what is not.
One of our largest responsibilities in the Emergency Department is sorting out benign from surgical or medical causes of abdominal pain. Morbidity and mortality varies by age and condition.
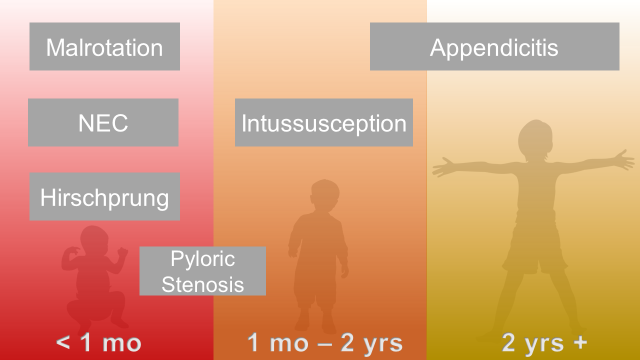
Abdominal Surgical Emergencies in Children: A Relative Timeline
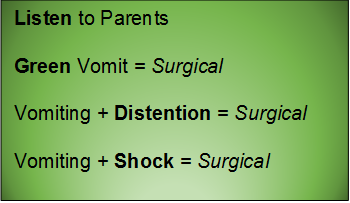
General Advice
Neonate (birth to one month)
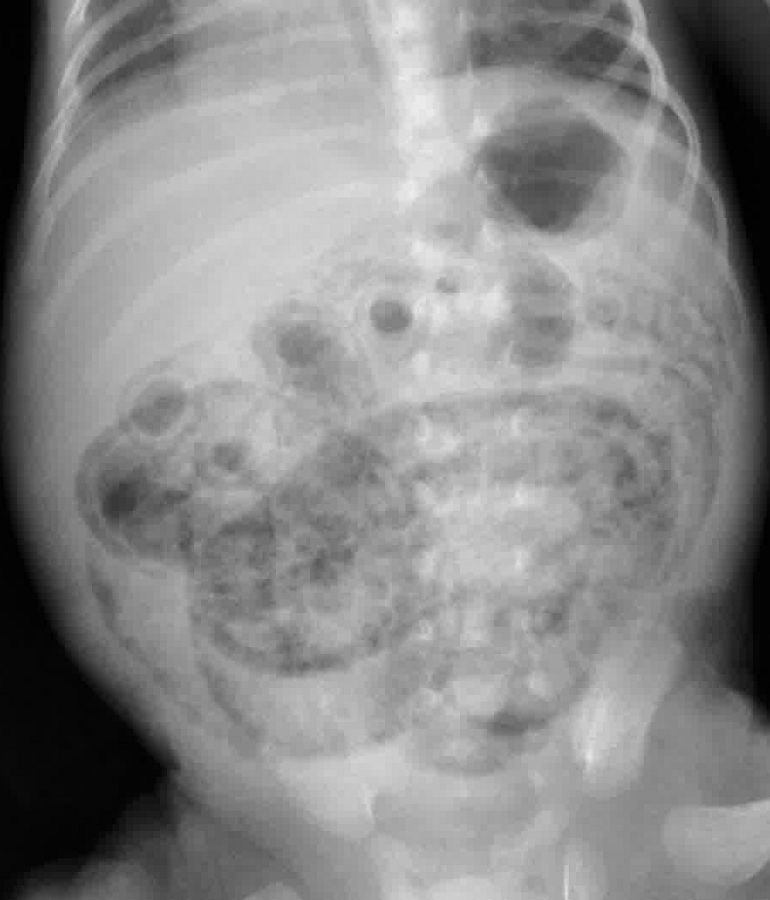
Necrotizing Enterocolitis
Essentials:
- Typically presents in 1st week of life (case reports to 6 months in chronically ill children)
- Extend suspicion longer in NICU graduates
- Up to 10% of all cases of necrotizing enterocolitis are in full-term children
- Pathophysiology is unknown, but likely a translocation of bacteria
Diagnosis:
- Feeding intolerance, abdominal distention
- Abdominal XR: pneumatosis intestinalis
Management:
- IV access, NG tube, broad-spectrum antibiotics, surgery consult, ICU admission
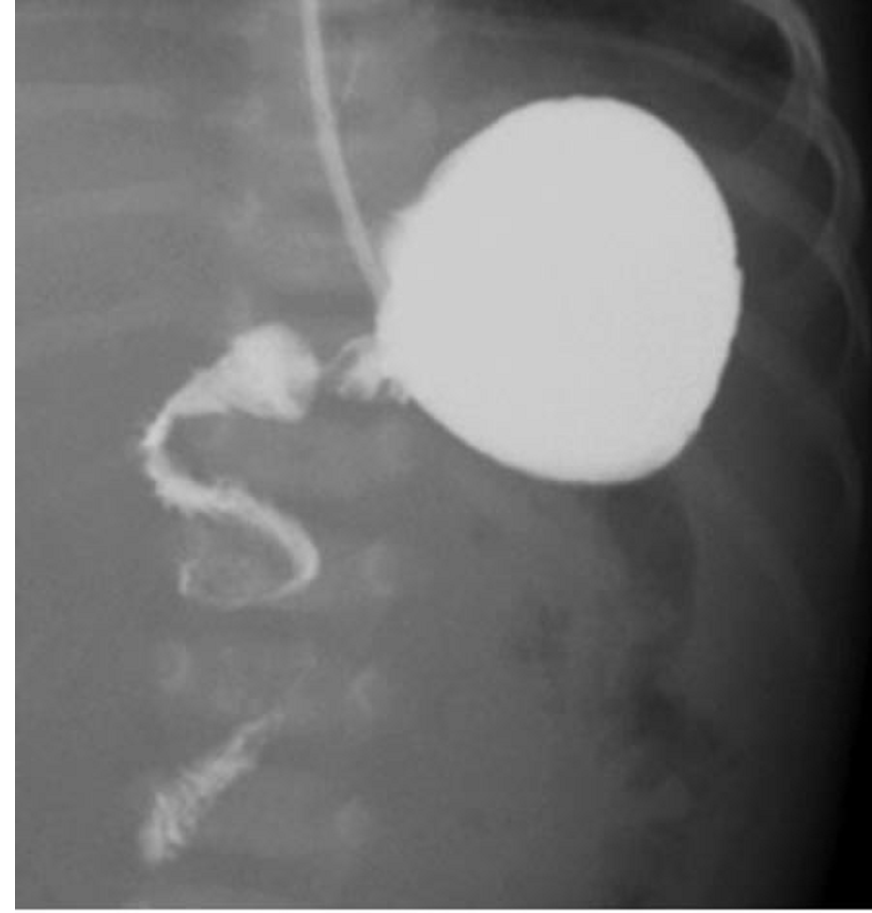
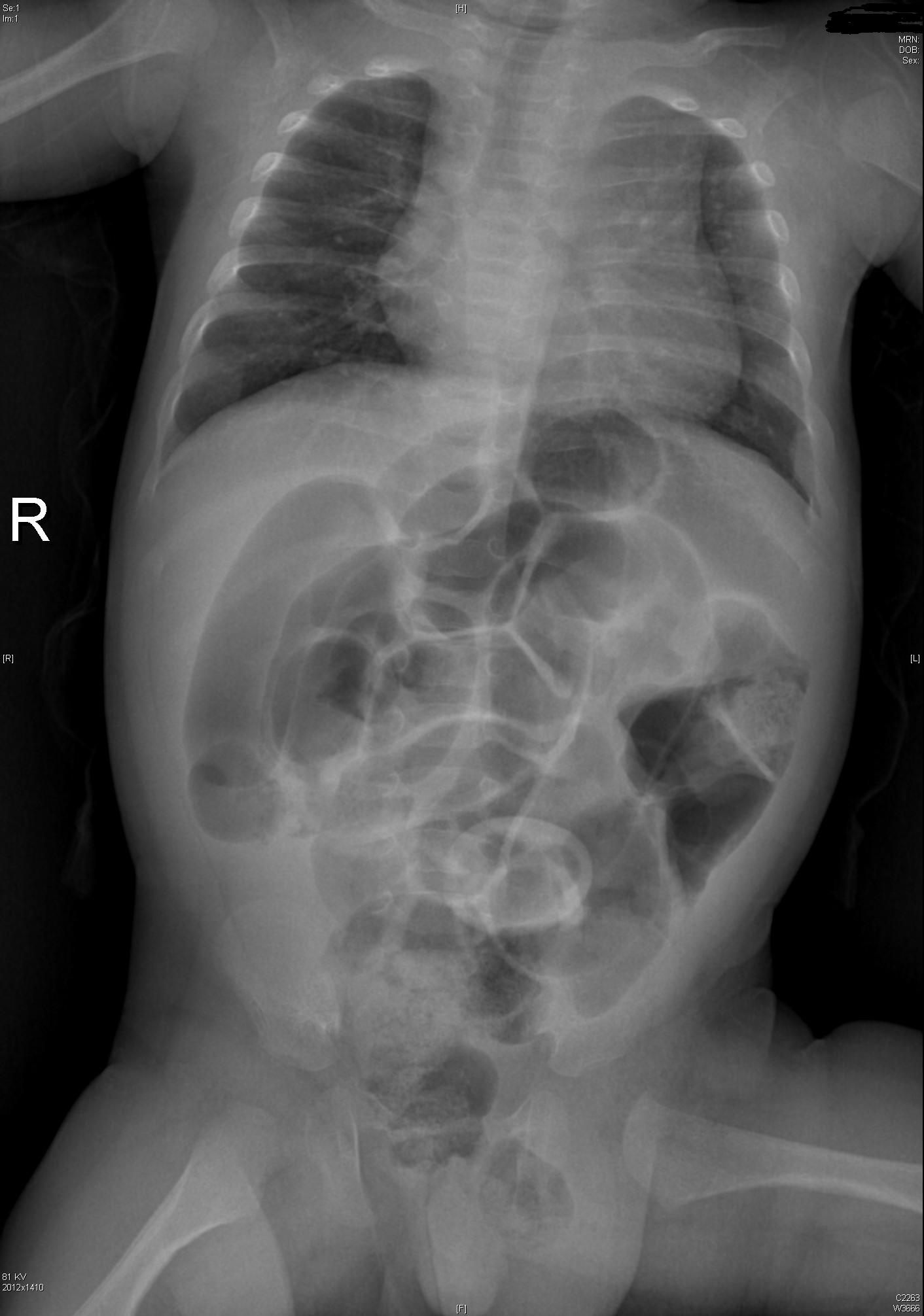
Intestinal Malrotation with Volvulus
Essentials:
- Bilious vomiting (80-100%) in the 1st month; especially in the 1st week
- May look well initially, then rapidly present in shock
- Ladd’s bands: abnormally high tethering of cecum to abdominal wall; peristalsis, volvulus, ischemia
Diagnosis:
- History of bilious emesis is sufficient to involve surgeons
- Upper GI series: corkscrew appearance
- US (if ordered) may show abnormal orientation of and/or flow to superior mesenteric artery and vein
Management:
- Stat surgical consult
- IV access, resuscitation, NG tube to decompress (bowel wall perfusion at risk, distention worsens)
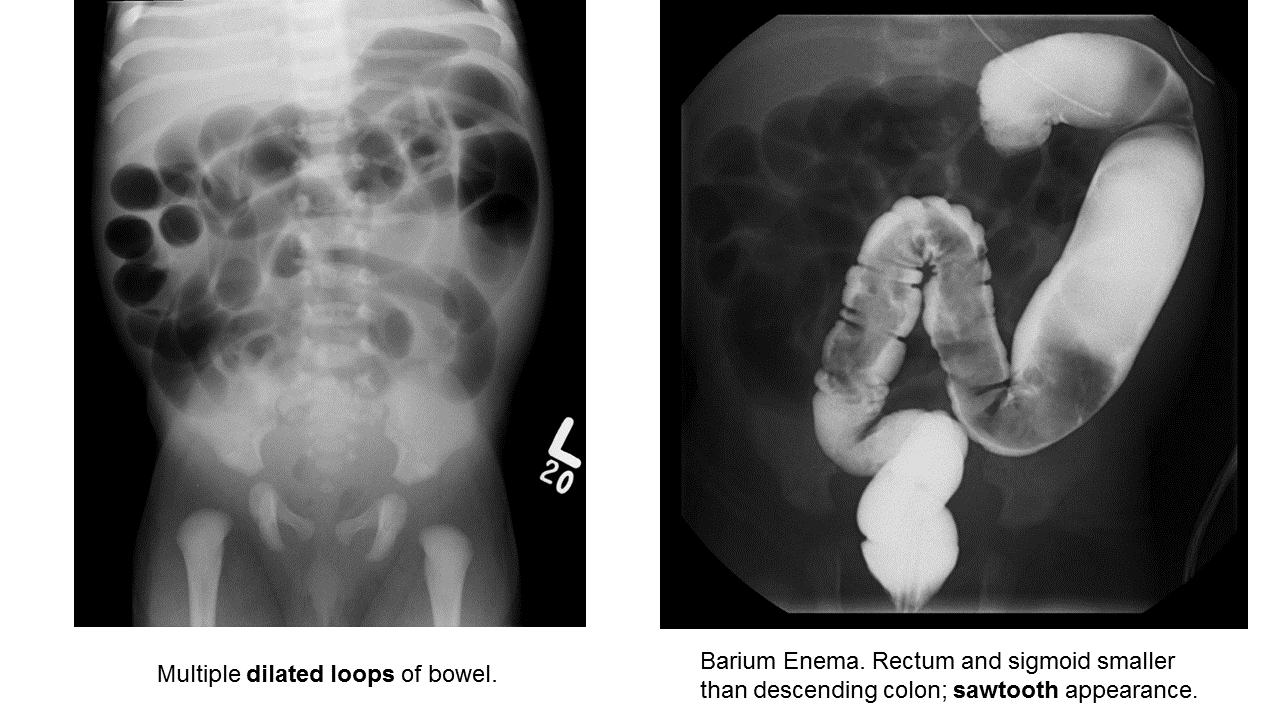
Hirschprung Disease
Essentials:
- Problem in migration of neural crest cells
- Aganglionic colon (80% rectosigmoid; 15-20% proximal to sigmoid; 5% total colonic aganglionosis) colon (known as short-segment disease)
- Poor to no peristalsis: constipation, perforation, and/or sepsis
Diagnosis:
- May be diagnosed early as “failure to pass meconium in 1st 48 hours”
- In ED, presents as either bowel obstruction or enterocolitis
- Contrast enema
- Beware of the toxic megacolon (vomiting, distention, sepsis)
Management:
- Resuscitation, antibiotics, NG tube decompression, surgical consultation; stable patients may need rectal biopsy for confirmation
- Staged surgery (abdominoperineal pull-through with diverting colostomy, subsequent anastomosis) versus one-stage repair.
Infant and Toddler (1 month to 2 years)
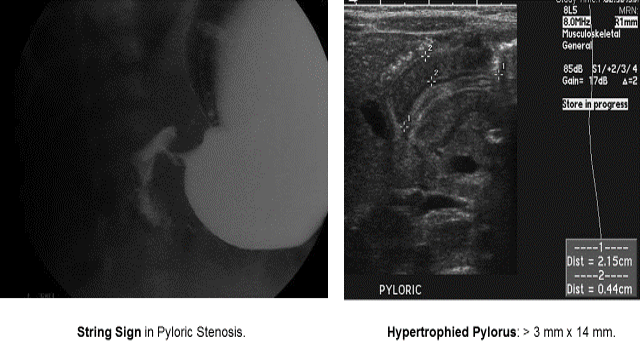
Pyloric Stenosis
Essentials:
- Hypertrophy of pyloric sphincter; genetic, environmental, exposure factorsString Sign in Pyloric Stenosis.
Diagnosis:
- Hungry, hungry, not-so-hippos; they want to eat all of the time, but cannot keep things down
- Poor weight gain (less than 20-30 g/day)
- US: “π–loric stenosis” (3.14); pylorus dimensions > 3 mm x 14 mm
- UGI: “string sign”
Management:
- Trial of medical treatment with oral atropine via NGT (muscarinic effects decrease pyloric tone)
- Ramstedt pyloromyotomy (definitive)
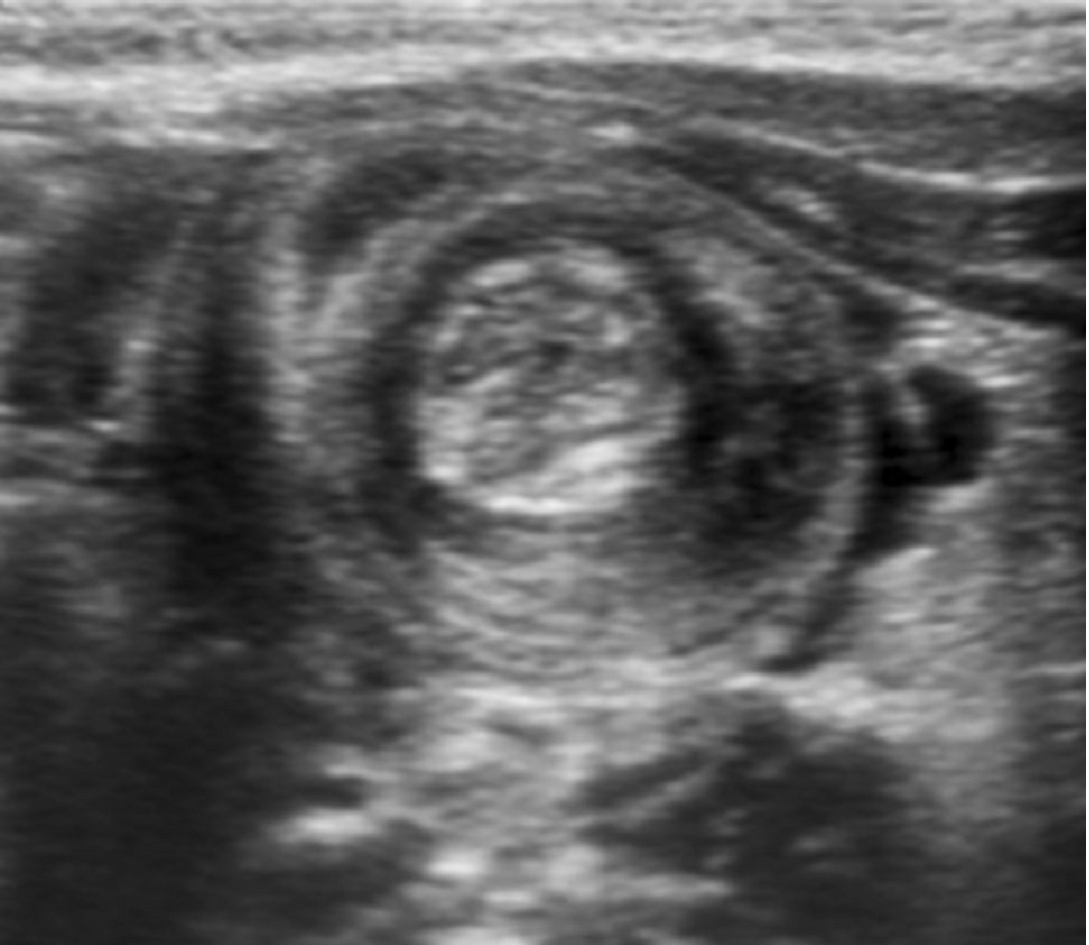
Intussusception
Essentials:
- Majority (90%) ileocolic; no pathological lead point
- Small minority (4%) ileoileocolic due to lead point: Meckel’s diverticulum, polyp, Peyer’s patches, Henoch-Schönlein purpura (intestinal hematoma)
Diagnosis:
- Ultrasound sensitivity and specificity near 100% in experienced hands
- Abdominal XR may show non-specific signs; used mainly to screen for perforation before reduction
Management:
- Hydrostatic enema: contrast (barium or water-soluble contrast with fluoroscopy) or saline (with ultrasound)
- Air-contrast enema: air or carbon dioxide (with either fluoroscopy or ultrasound); higher risk for perforation than hydrostatic (1% risk), but generally safer than perforation from contrast
- Consider involving surgical service early (precaution before reduction)
- Traditional disposition is admission; controversial: home discharge from ED
Young Child and Older (2 years and up)
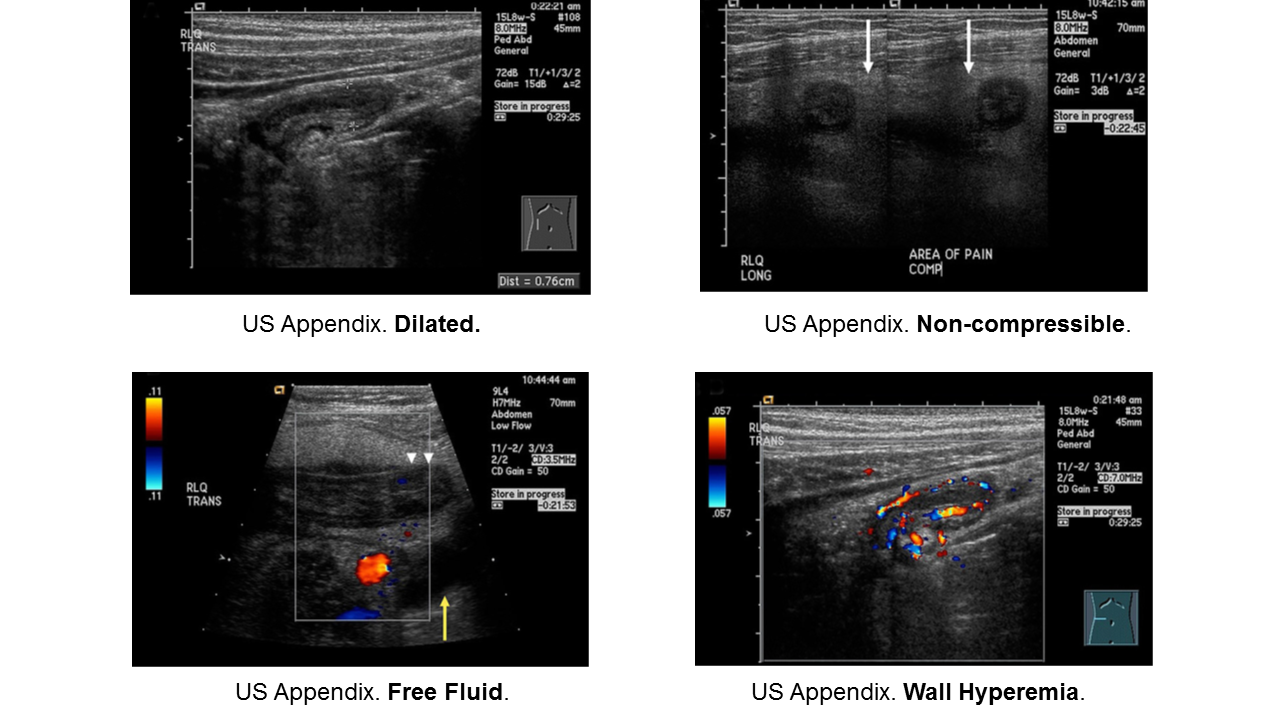
Appendicitis
Essentials:
- Appendicitis occurs in all ages, but rarer in infants. Infants do not have fecalith; rather they have some other anatomic or congenital condition.
- More common in school-aged children (5-12 years) and adolescents
- Younger children present atypically, more likely to have perforated when diagnosed.
Diagnosis:
- Non-specific signs and symptoms
- Often have abdominal pain first; vomiting comes later
- Location/orientation of appendix varies
- Appendicitis scores vary in their performance
- Respect fever and abdominal pain
Management:
- Traditional: surgical
- On the horizon: identification of low-risk children who may benefit from trial of antibiotics
- If perforated, interval appendectomy (IV antibiotics via PICC for 4-6 weeks, then surgery)
Obstruction
Essentials:
- Same pathophysiology and epidemiology as adults: “ABC” – adhesions, “bulges” (hernias), and cancer.
Diagnosis:
- Obstruction is a sign of another condition. Look for cause of obstruction: surgical versus medical
- Abdominal XR in low pre-test probability
- CT abdomen/pelvis for moderate-to-high risk; confirmation and/or surgical planning
Management:
- Treat underlying cause
- NG tube to low intermittent wall suction
- Admission, fluid management, serial examinations
Take these pearls home:
- Consider surgical pathology early in encounter
- Resuscitate while you investigate
- Have a low threshold for imaging and/or consultation, especially in preverbal children
Selected References
Necrotizing Enterocolitis
Neu J, Walker A. Necrotizing Enterocolitis. N Eng J Med. 2011; 364(3):255-264.
Niño DF et al. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nature. 2016; 13:590-600.
Walsh MC et al. Necrotizing Enterocolitis: A Practitioner’s Perspective. Pediatr Rev. 1988; 9(7):219-226.
Malrotation with Midgut Volvulus
Applegate KE. Intestinal Malrotation in Children: A Problem-Solving Approach to the Upper Gastrointestinal Series. Radiographics. 2006; 26:1485-1500.
Kapfer SA, Rappold JF. Intestinal Malrotation – Not Just the Pediatric Surgeon’s Problem. J Am Coll Surg. 2004; 199(4):628-635.
Lee HC et al. Intestinal Malrotation and Catastrophic Volvulus in Infancy. J Emerg Med. 2012; 43(1):49-51.
Martin V, Shaw-Smith C. Review of genetic factors in intestinal malrotation. Pediatr Surg Int. 2010; 26:769-781.
Nehra D, Goldstein AM. Intestinal malrotation: Varied clinical presentation from infancy through adulthood. Surgery. 2010; 149(3):386-391.
Hirschprung Disease
Amiel J, Sproat-Emison E, Garcia-Barcelo M, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet 2008; 45:1.
Arshad A, Powell C, Tighe MP. Hirschsprung's disease. BMJ 2012; 345:e5521.
Aworanti OM, McDowell DT, Martin IM, Quinn F. Does Functional Outcome Improve with Time Postsurgery for Hirschsprung Disease? Eur J Pediatr Surg 2016; 26:192.
Clark DA. Times of first void and first stool in 500 newborns. Pediatrics 1977; 60:457.
Dasgupta R, Langer JC. Evaluation and management of persistent problems after surgery for Hirschsprung disease in a child. J Pediatr Gastroenterol Nutr 2008; 46:13.
De Lorijn F, Reitsma JB, Voskuijl WP, et al. Diagnosis of Hirschsprung's disease: a prospective, comparative accuracy study of common tests. J Pediatr 2005; 146:787.
Doig CM. Hirschsprung's disease and mimicking conditions. Dig Dis 1994; 12:106.
Khan AR, Vujanic GM, Huddart S. The constipated child: how likely is Hirschsprung's disease? Pediatr Surg Int 2003; 19:439.
Singh SJ, Croaker GD, Manglick P, et al. Hirschsprung's disease: the Australian Paediatric Surveillance Unit's experience. Pediatr Surg Int 2003; 19:247.
Suita S, Taguchi T, Ieiri S, Nakatsuji T. Hirschsprung's disease in Japan: analysis of 3852 patients based on a nationwide survey in 30 years. J Pediatr Surg 2005; 40:197.
Sulkowski JP, Cooper JN, Congeni A, et al. Single-stage versus multi-stage pull-through for Hirschsprung's disease: practice trends and outcomes in infants. J Pediatr Surg 2014; 49:1619.
Pyloric Stenosis
Aspelund G, Langer JC. Current management of hypertrophic pyloric stenosis. Semin Pedaitr Surg. 2007; 16:27-33.
Dias SC et al. Hypertrophic pyloric stenosis: tips and tricks for ultrasound diagnosis. Insights Imaging. 2012; 3:247-250.
Kawahara H et al. Medical treatment of infantile hypertrophic pyloric stenosis: should we always slice the olive? J Pediatr Surg. 2005; 40:1848-1851.
Mack HC. Adult Hypertrophic Pyloric Stenosis. Arch Inter Med. 1959; 104:78-83.
Meissner PE et al. Conservative treatment of infantile hypertrophic pyloric stenosis with intravenous atropine sulfate does not replace pyloromyotomy. Pediatr Surg Int. 2006; 22:1021-1024.
Mercer AE, Phillips R. Can a conservative approach to the treatment of hypertrophic pyloric stenosis with atropine be considered a real alternative to pyloromyotomy? Arch Dis Child. 2013; 95(6): 474-477.
Pandya S, Heiss K, Pyloric Stenosis in Pediatric Surgery.Surg Clin N Am. 2012; 92:527-39.
Peters B et al. Advances in infantile hypertrophic pyloric stenosis. Expert Rev Gastroenterol Hepatol. 2014; 8(5):533-541.
Intussusception
Apelt N et al. Laparoscopic treatment of intussusception in children: A systematic review. J Pediatr Surg. 2013; 48:1789-1793.
Applegate KE. Intussusception in Children: Imaging Choices. Semin Roentgenol. 2008; 15-21.
Bartocci M et al. Intussusception in childhood: role of sonography on diagnosis and treatment. J Ultrasound. 2015; 18 Gilmore AW et al. Management of childhood intussusception after reductiion by enema. Am J Emerg Med. 2011; 29:1136-1140.:205-211.
Chien M et al. Management of the child after enema-reduced intussusception: hospital or home? J Emerg Med. 2013; 44(1):53-57.
Cochran AA et al. Intussusception in traditional pediatric, nontraditional pediatric, and adult patients. Am J Emerg Med. 2011; 523-527.
Loukas M et al. Intussusception: An Anatomical Perspective With Review of the Literature. Clin Anatomy. 2011; 24: 552-561.
Mendez D et al. The diagnostic accuracy of an abdominal radiograph with signs and symptoms of intussusception. Am J Emerg Med. 2012; 30:426-431.
Whitehouse et al. Is it safe to discharge intussusception patients after successful hydrostatic reduction? J Pediatr Surg. 2010; 45:1182-1186.
Appendicitis
Amin P, Chang D. Management of Complicated Appendicitis in the Pediatrc Population: When Surgery Doesn’t Cut it. Semin Intervent Radiol. 2012; 29:231-236
Blakely ML et al. Early vs Interval Appendectomy for Children With Perforated Appendicitis. Arch Surg. 2011; 146(6):660-665.
Bundy DG et al. Does This Child Have Appendicitis? JAMA. 2007; 298(4):438-451.
Cohen B et al. The non-diagnostic ultrasound in appendicitis: is a non-visualized appendix the same as a negative study? J Pediatr Surg. 2015 Jun;50(6):923-7
Herliczek TW et al. Utility of MRI After Inconclusive Ultrasound in Pediatric Patients with Suspected Appendicitis. AJT. 2013; 200:969-973.
Janitz et al. Ultrasound Evaluation for Appendicitis. J Am Osteopath Coll Radiol. 2016; 5(1):5-12.
Kanona H et al. Stump Appendicitis: A Review. Int J Surg. 2012; 10:4255-428.
Kao LS et al. Antibiotics vs Appendectomy for Uncomplicated Acute Appendicitis. Evid Based Rev Surg. 2013;216(3):501-505.
Petroianu A. Diagnosis of acute appendicitis. Int J Surg. 2012; 10:115-119.
Mazeh H et al. Tip appendicitis: clinical implications and management. Amer J Surg. 2009; 197:211-215.
Puig S et al. Imaging of Appendicitis in Children and Adolescents. Semin Roentgenol. 2008; 22-28.
Schizas AMP, Williams AB. Management of complex appendicitis. Surgery. 2010; 28(11):544-548.
Shogilev DJ et al. Diagnosing Appendicitis: Evidence-Based Review. West J Emerg Med. 2014; 15(4):859-871.
Wray CJ et al. Acute Appendicitis: Controversies in Diagnosis and Management. Current Problems in Surgery. 2013; 50:54-86
Intestinal Obstruction
Babl FE et al. Does nebulized lidocaine reduce the pain and distress of nasogastric tube insertion in young children? A randomized, double-blind, placebo-controlled trial. Pediatrics. 2009 Jun;123(6):1548-55
Chinn WM, Zavala DC, Ambre J. Plasma levels of lidocaine following nebulized aerosol administration. Chest 1977;71(3):346-8.
Cullen L et al. Nebulized lidocaine decreases the discomfort of nasogastric tube insertion: a randomized, double-blind trial. Ann Emerg Med. 2004 Aug;44(2):131-7.
Gangopadhyay AN, Wardhan H. Intestinal obstruction in children in India. Pediatr Surg Int. 1989; 4:84-87.
Hajivassiliou CA. Intestinal Obstruction in Neonatal/Pediatric Surgery. Semin Pediatr Surg. 2003; 12(4):241-253.
Hazra NK et al. Acute Intestinal Obstruction in children: Experience in a Tertiary Care Hospital. Am J Pub Health Res. 2015; 3(5):53-56.
Kuo YW et al. Reducing the pain of nasogastric tube intubation with nebulized and atomized lidocaine: a systematic review and meta-analysis. J Pain Symptom Manage. 2010 Oct;40(4):613-20. .
Pediatric Surgery
Irish MS et al. The Approach to Common Abdominal Diagnoses in Infants and Children. Pedaitr Clin N Am. 1998; 45(4):729-770.
Louie JP. Essential Diagnosis of Abdominal Emergencies in the First Year of Life. Emerg Med Clin N Am. 2007; 25:1009-1040.
McCullough M, Sharieff GQ. Abdominal surgical emergencies in infants and young children. Emerg Med Clin N Am. 2003; 21:909-935.
Pepper VK et al. Diagnosis and Management of Pediatric Appendicitis, Intussusception, and Meckel Diverticulum. Surg Clin N Am. 2012
This post and podcast are dedicated to Mr Ross Fisher for his passion and spirit of collaboration in all things #FOAMed. Thank you, sir!
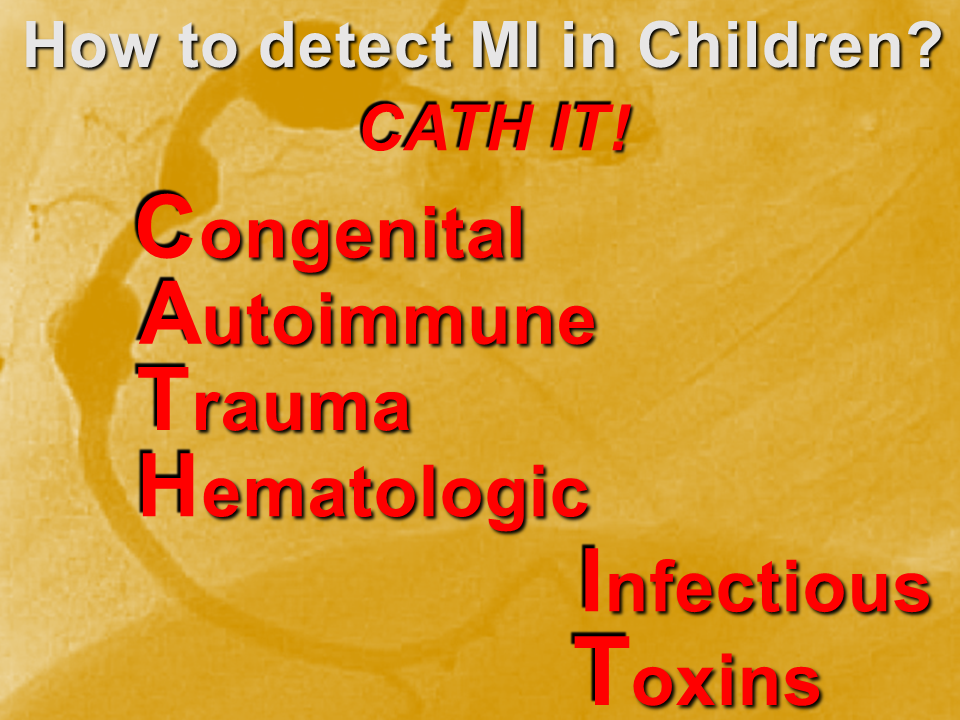
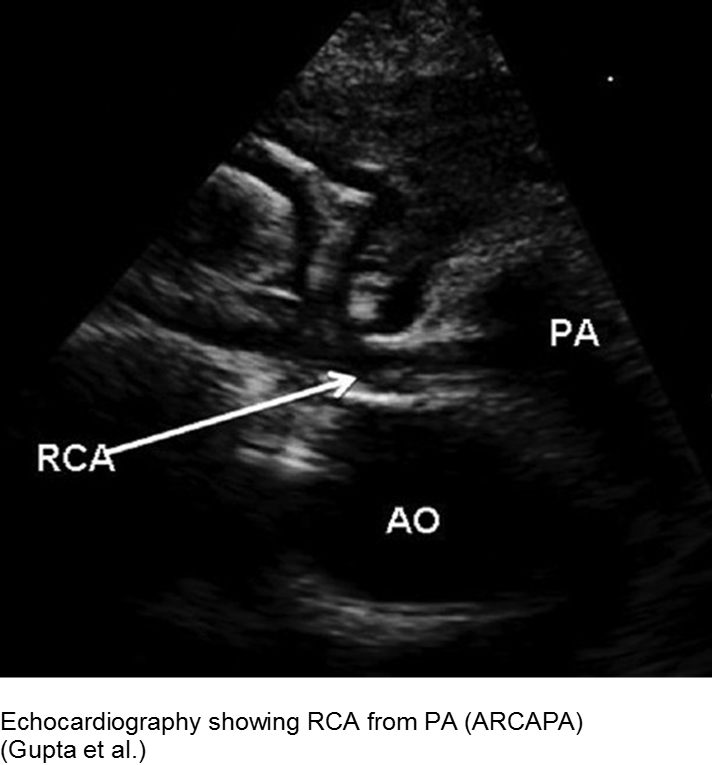
Myocardial infarction (MI) in children is uncommon, but underdiagnosed. This is due to two main factors: the etiologies are varied; and the presenting symptoms are “atypical”.
We need a mental metal detector!
Case examples
Congenital
Two main presentations of MI due to congenital lesions: novel and known. The novel presentation is at risk for underdiagnosis, due to its uncommonness and vague, atypical symptoms. There are usually some red flags with a careful H&P. The known presentation is a child with a history of congenital heart disease, addressed by corrective or palliative surgery. This child is at risk for expected complications, as well as overdiagnosis and iatrogenia. Risk stratify, collaborate with specialists.
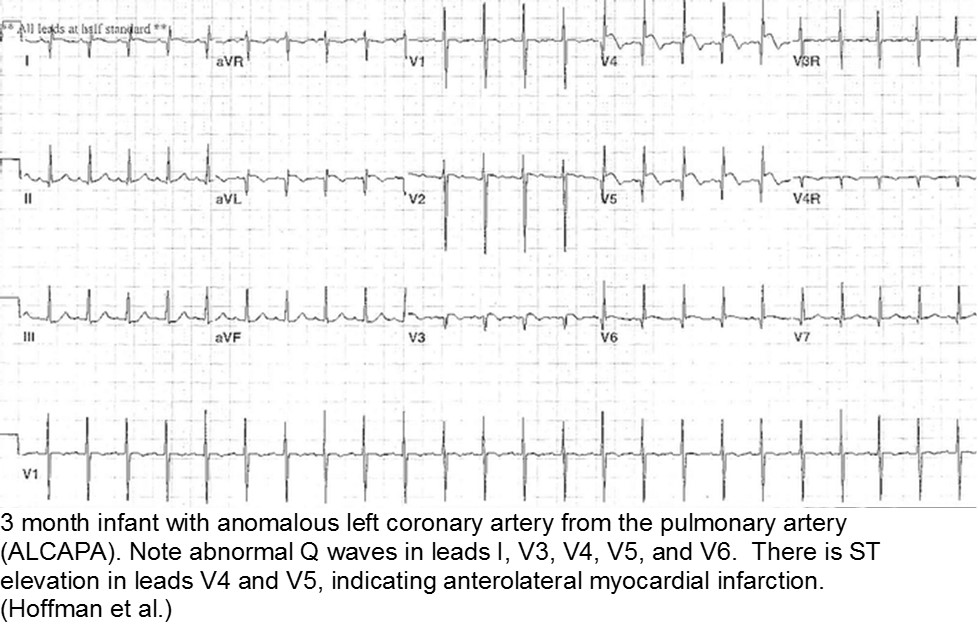
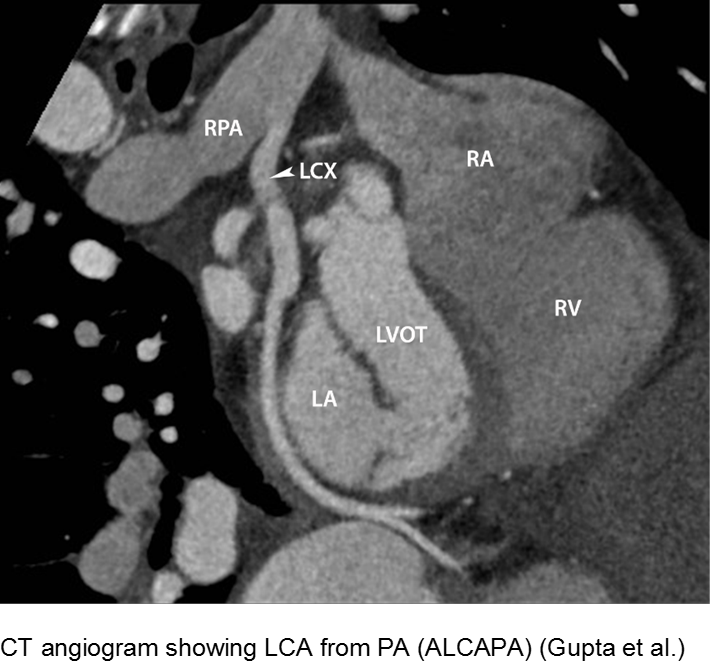
The fussy, sweaty feeder: ALCAPA
Anomalous Left Coronary Artery from the Pulmonary Artery (ALCAPA) is an example of what can go wrong during fetal development: any abnormality in the number, origin, course, or morphology of the coronary arteries can present as a neonate with sweating during feeds (steal syndrome), an infant in CHF, or an older child with failure to thrive or poor exercise tolerance.
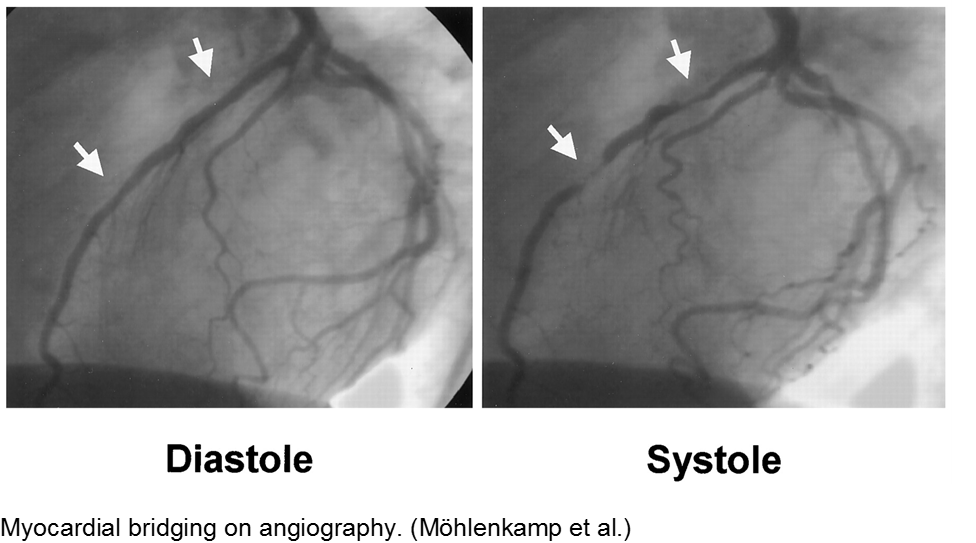
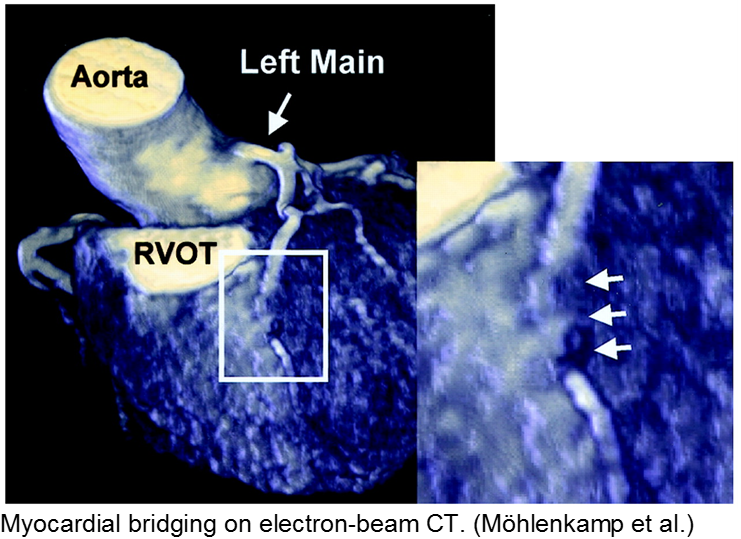
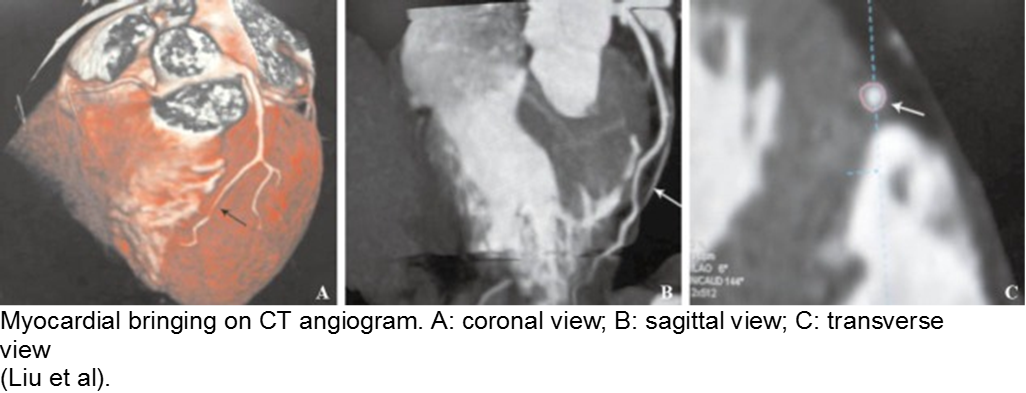
The stable child with chest pain: myocardial bridge
Normal coronary arteries run along the epicardial surface of the heart, with projections into the myocardium. If part of the artery’s course runs within the myocardium (i.e. the artery weaves into and/or out of the myocardium), then there is a myocardial bridge of the coronary artery. With every systolic contraction, the artery is occluded.
Although a myocardial bridge may not cause symptoms (especially at distal portions), the area it supplies is at risk.
With any minor trauma or exertion, demand may outpace supply, resulting in ischemia.
Diagnosis is made on coronary angiography.
The unwell child post-cardiac surgery: Fontan problems
The child with single ventricle physiology may have a Norwood procedure at birth (creation of a neoaorta, atrial septectomy, and Blalock-Taussig shunt), a Bidirectional Glenn procedure at 3-6 months (shunt removed, superior vena cava connected to pulmonary arteries), and a Fontan procedure at about 2-3 years of age (inferior vena cava blood flow is shunted into the pulmonary arteries).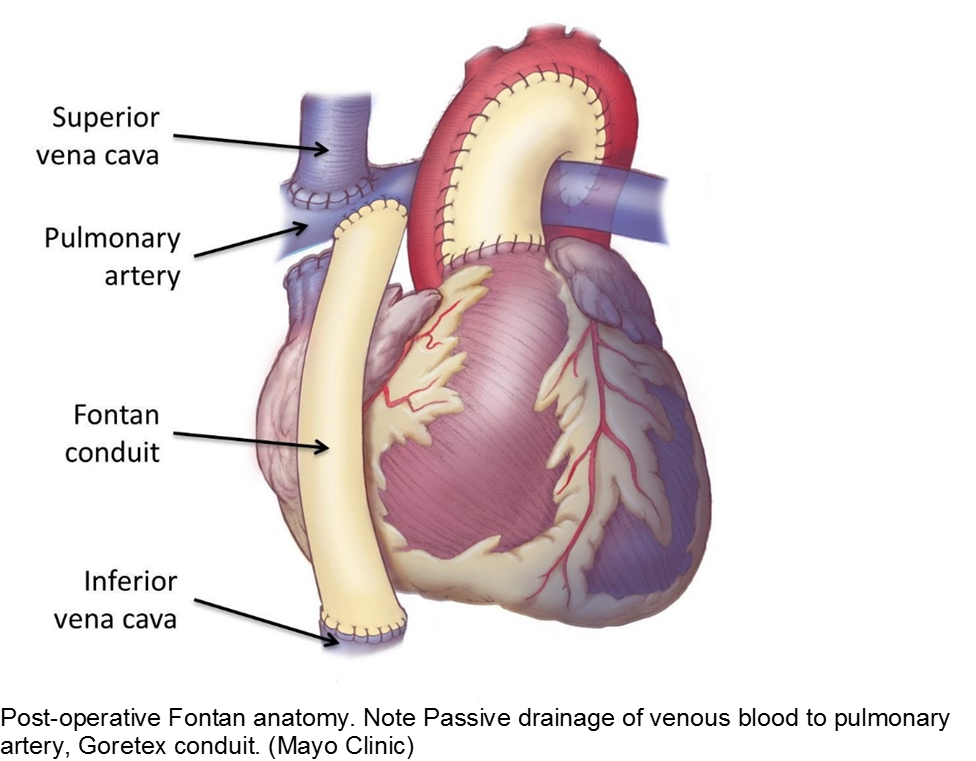
These children depend on their preload to run blood passively into the pulmonary circuit; afterload reduction is also important to compensate for a poor left ejection fraction, as well as to avoid the development of pulmonary hypertension. They are typically on an anticoagulant (often aspirin), a diuretic (e.g. furosemide), and an afterload reduction agent (e.g. enalapril).
Any disturbance in volume status (hyper- or hypovolemia), anticoagulation, or afterload may cause myocardial strain or infarction. Take the child s/p Fontan seriously and involve his specialists early with any concerns.
Autoimmune
The body’s inflammatory-mediated reaction to a real or perceived insult can cause short- and long-term cardiac sequelae. Find out how well the underlying disease is controlled, and what complications the child has had in the past.
The red, hot, crispy, flaky child: acute Kawasaki disease
Kawasaki disease (KD) is an acute systemic vasculitis, diagnosed by the presence of fever for five or more days accompanied by four or more criteria: bilateral conjunctival injection, mucositis, cervical lymphadenopathy, polymorphous rash, and palmar or sole desquamation. The criteria may occur (and disappear) at any time during the illness.
Infants are under double jeopardy with Kawasaki Disease. They are more likely to have incomplete KD (i.e. not fulfill strict criteria) and if they have KD, they are more likely to suffer the dangerous consequences of aneurysm formation (chiefly coronary arteries, but also brain, kidney). Have a low threshold for investigation.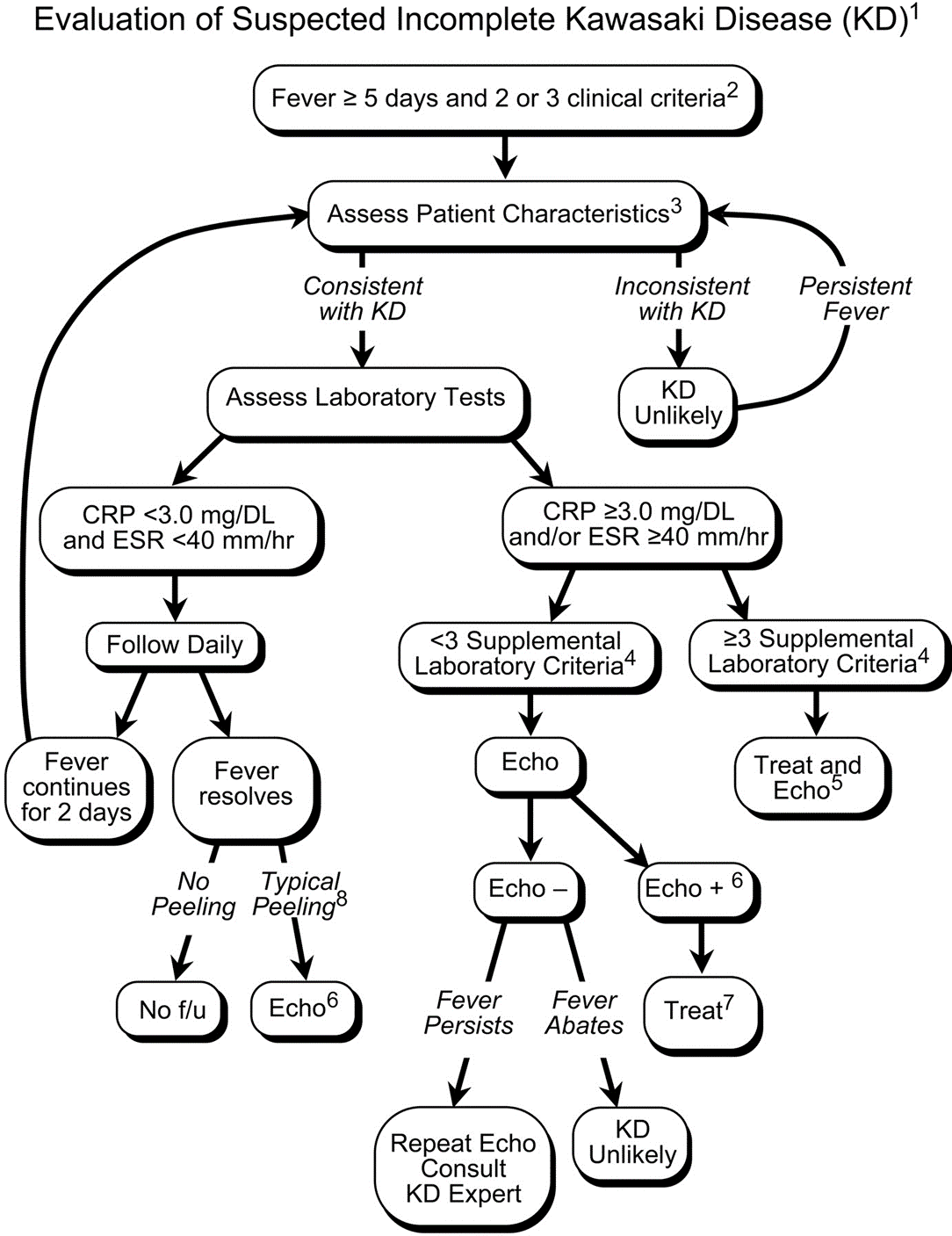
Treatment includes 2 g/kg/day IVIG and high-dose aspirin (30-50 mg/kg/day) acutely, then low-dose aspirin (5 mg/kg/day) for weeks to months. Regular and long-term follow-up with Cardiology is required.
The aftermath: sequelae of Kawasaki disease
The family and child with a history of KD may have psychological trauma and continuous anxiety about the child’s risk of MI. Approximately 4.7% of children who were promptly diagnosed and correctly treated will go on to have cardiac sequelae.
Children who have no detected cardiac sequelae by 8 weeks, typically continue to be asymptomatic up to 20 years later.
Smaller aneurysms tend to regress over time, especially those < 6 mm.
Thrombi may calcify, or the lumen may become stenotic due to myofibroblast proliferation. Children with any coronary artery dilatation from KD should be followed indefinitely.
Giant aneurysms (≥8 mm) connote the highest risk for MI.
Parents often are concerned about recurrence, and any subsequent fever can be distressing. There is a low rate of recurrence for KD: approximately 2%. Infants who have coronary aneurysms are at the highest risk for recurrence.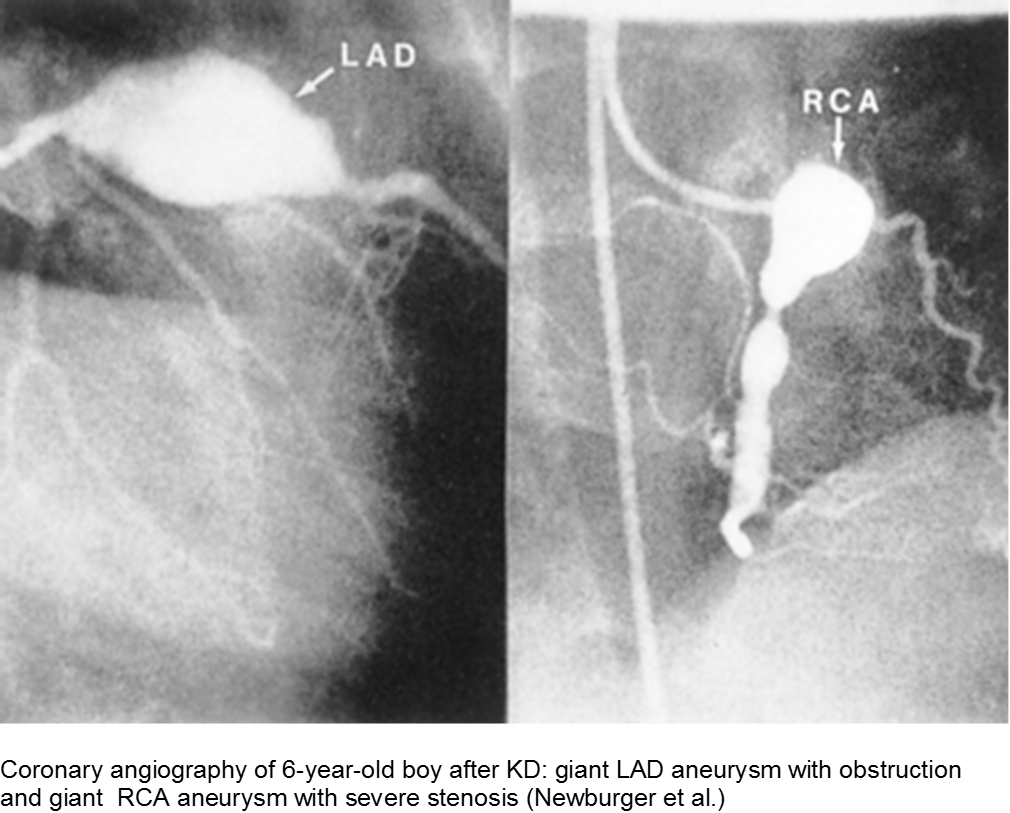
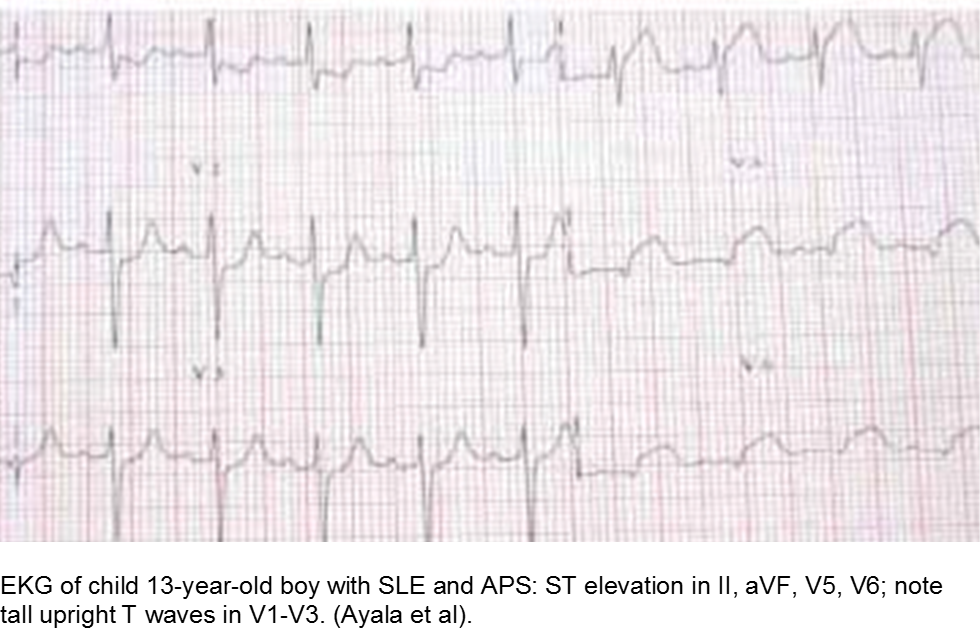
The older child with vague chest complaints and hypercoagulability: Systemic Lupus Erythematosus and Anti-Phospholipid Syndrome
Up to 15% of cases of SLE begin in childhood. Adult criteria are used, with the caveat that the diagnosis of SLE in children can be challenging; many children only manifest a few of the criteria initially before going on to develop further systemic involvement.
The Systemic Lupus International Collaborating Clinics (SLICC) revised the criteria in 2012. The patient should have ≥4/17 clinical and/or immunologic criteria. The clinical criteria are: acute cutaneous (malar); chronic cutaneous (discoid); oral; alopecia; synovitis; serositis; renal; neurologic; hemolytic anemia; leukopenia; or thrombocytopenia. The immunologic criteria are: ANA; anti-dsDNA; anti-Sm; antiphospholipid; low complement; and/or Direct Coombs (in absence of hemolytic anemia). At least one criterion should be clinical, and at least one should be immunologic.
Children with antiphospholipid syndrome (APS) may occur with or without SLE. Patients are at risk for venous and arterial thrombi formation. APS may also cause structural damage, such as valvular thickening and valvular nodes (Libman-Sacks endocarditis). Mitral and aortic valves are at the highest risk.
Although most children with chest pain will not have MI, those with comorbidities should be investigated carefully.
Trauma
Direct, blunt trauma to the chest can cause myocardial stunning, dysrhythmias, or an asymptomatic rise in Troponin I. However, some children are at risk for disproportionate harm due to a previously unknown risk factor. Clinically significant cardiac injury occurs in up to 20% of patients with non-penetrating thoracic trauma.
The motor vehicle collision: blunt myocardial injury
Direct trauma (steering wheel, airbag, seatbelt), especially in fast acceleration-deceleration injury, may cause compression of the heart between the sternum and the thoracic spine.
Electrocardiography (ECG) should be performed on any patient with significant blunt chest injury. A negative ECG is highly consistent with no significant blunt myocardial injury.
Any patient with a new abnormality on ECG (dysrhythmia, heart block, or signs of ischemia) should be admitted for continuous ECG monitoring.
Elevation in troponin is common, but not predicted. A solitary elevated troponin without ECG abnormality is of unclear significance. Author’s advice: obtain troponin testing if there is an abnormal ECG, more than fleeting suspicion of BCI, and/or the child will be admitted for monitoring.
Hemodynamically labile children should be resuscitated and a stat transesophageal echocardiogram obtained.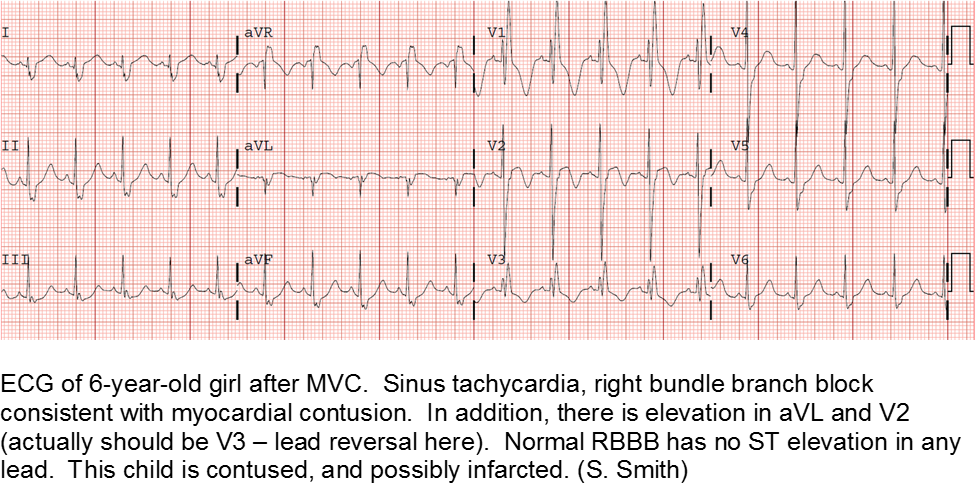
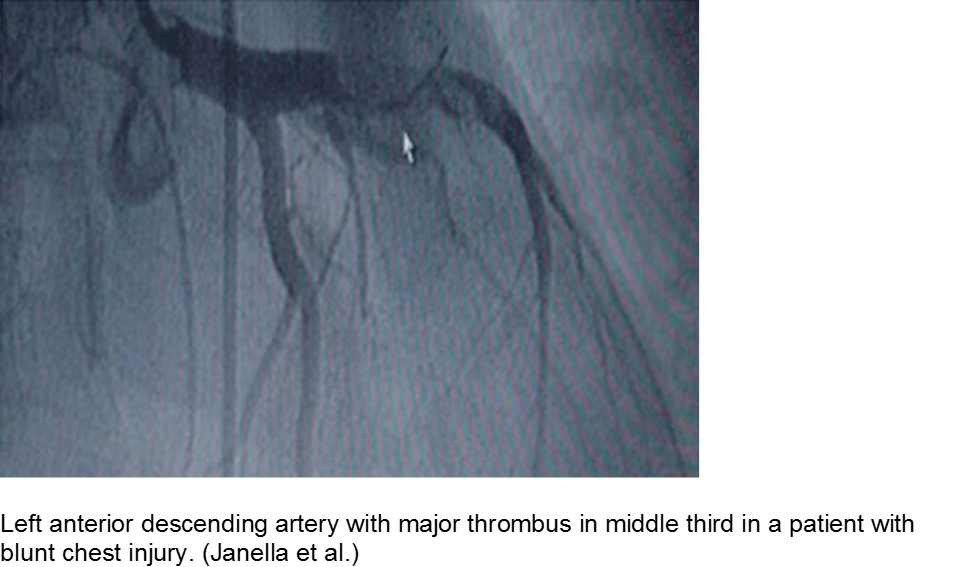
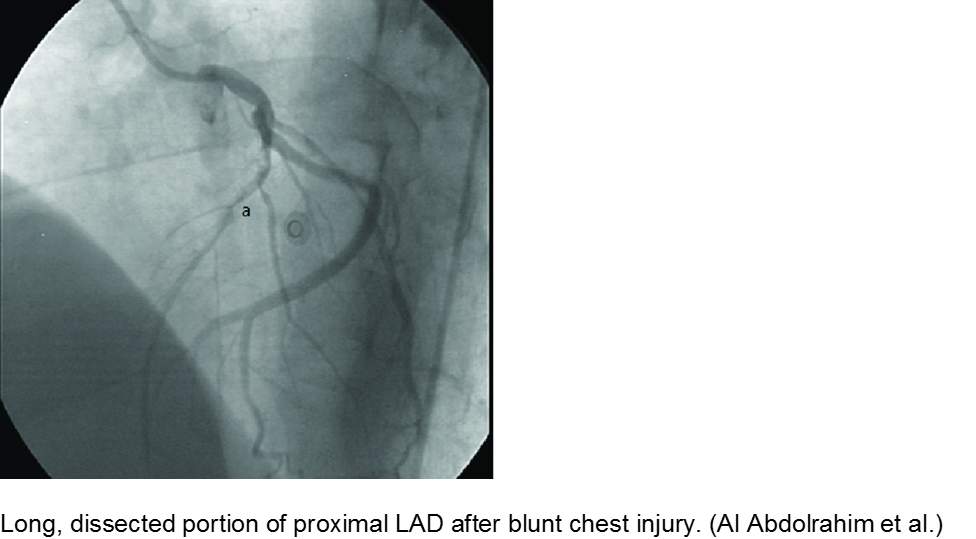
The high-velocity object: coronary artery dissection or thrombus
Direct trauma (e.g. MVC, baseball, high-velocity soccer ball) may cause damage to the left anterior descending artery or left circumflex artery, at the highest risk due to their proximity to the chest wall. Thrombosis and/or dissection may result, often presenting in a focal pattern of ischemia on the ECG.
Echocardiography may reveal valvular damage related to the injury, as well as effusion and ejection fraction. Since there is often a need to investigate the coronary anatomy, percutaneous coronary intervention (PCI) is recommended.
The minor trauma with disproportionate complaint: myocardial bridge
As mentioned in the congenital section (above), a known variation of a coronary artery’s course involves weaving in and out of the myocardium, creating a baseline risk for ischemia. Even minor trauma in a child with a myocardial bridge may cause acute thrombus, or slow stenosis from resulting edema. Unfortunately, the presence of myocardial bridging is often unknown at the time of injury. Approximately 25% of the population may have myocardial bridging, based on autopsy studies. Take the child seriously who has disproportionate symptoms to what should be a minor injury.
Hematologic
Coagulopathic and thrombophilic states may predispose children to focal cardiac ischemia. The best documented cormorbidity is sickle cell disease, although other pro-thrombotic conditions also put the child at risk.
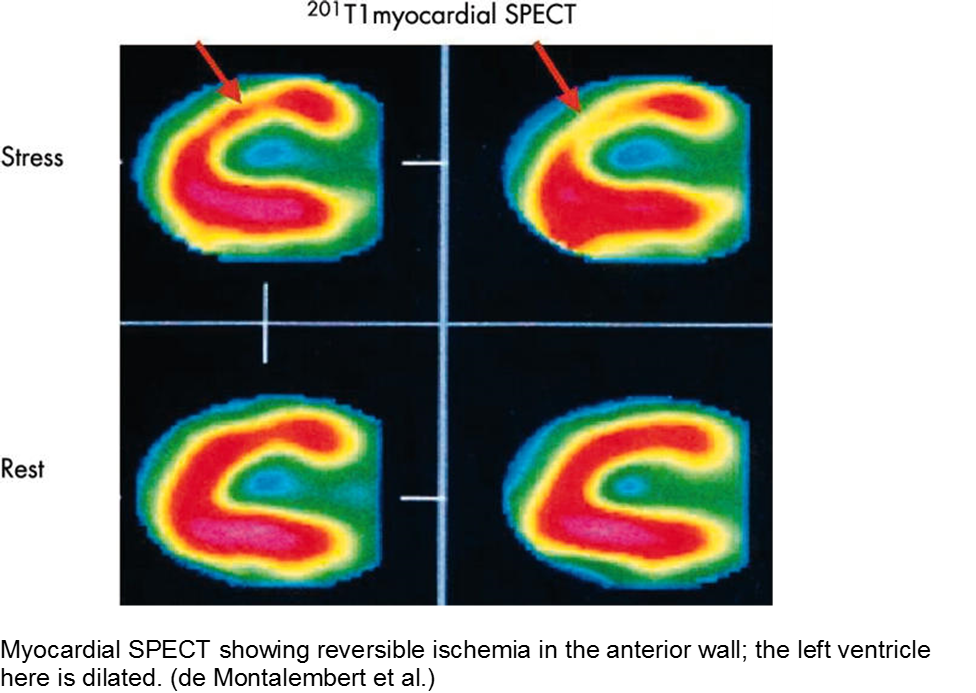
The child with sickle cell disease and chest pain: when it’s not acute chest syndrome
Sickle cell disease (SCD) can affect any organ system, although the heart is traditionally considered a lower-risk target organ for direct sickling and ischemia. The major cardiac morbidity in sickle cell is from strain, high-output failure and multiple, serial increases in myocardial demand, causing left ventricular hypertrophy and congestive heart failure.
However, there is mounting evidence that acute myocardial ischemia in sickle cell disease may be underappreciated and/or attributed to other causes of chest pain.
Other cardiac sequelae from SCD include pulmonary hypertension, left ventricular dysfunction, right ventricular dysfunction, and chronic iron overload.
Evidence of myocardial ischemia/infarction in children with SCD has been demonstrated on single-photon emission computed tomography (SPECT) scan.
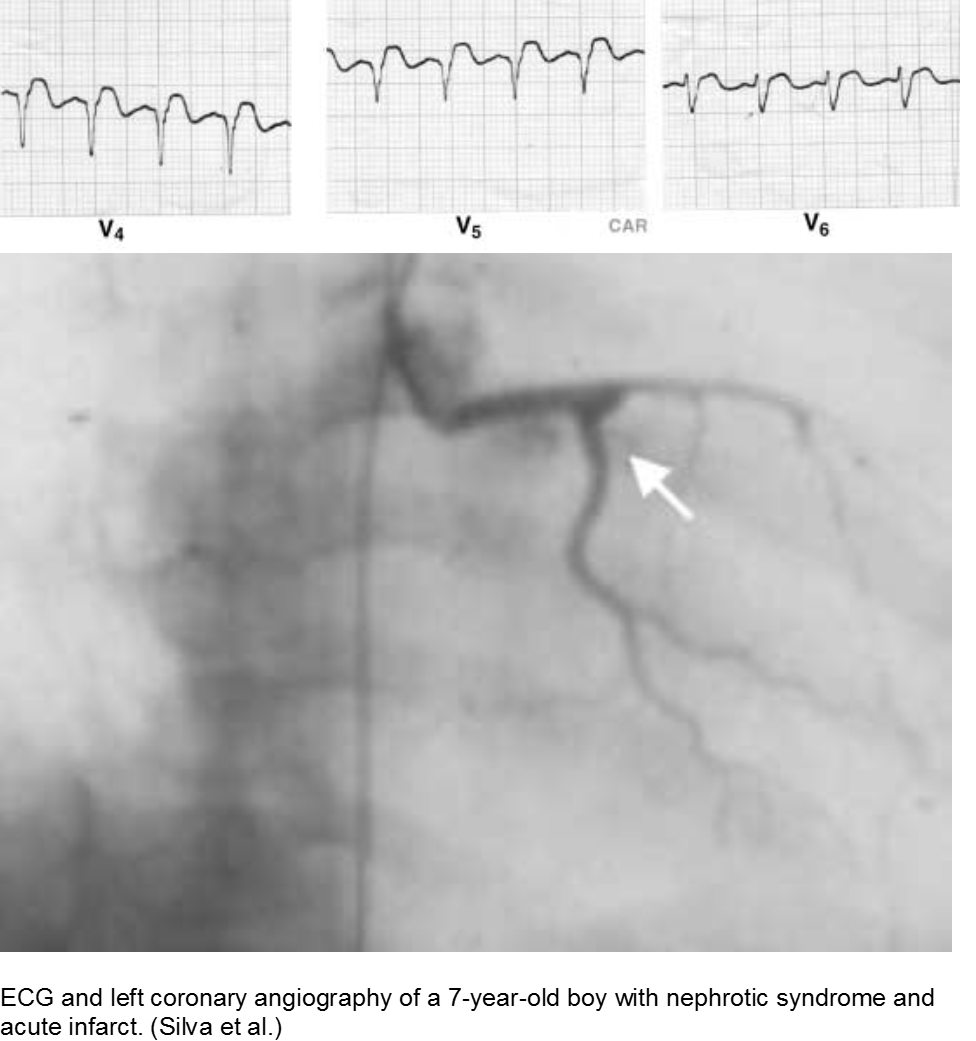
The puffy faced child with chest pain: nephrotic syndrome hypercoagulability
Children who suffer from nephrotic syndrome lose proteins that contribute to the coagulation cascade. In addition, lipoprotein profiles are altered: there is a rise in the very low-density lipoproteins (LDL), contributing to accelerated atherosclerosis. Typically nephrotic patients have normal levels of high-density lipoproteins (HDL), unless there is profuse proteinuria.
Children with difficult-to-control nephrotic syndrome (typically steroid-resistant) may form accelerated plaques that rupture, causing focal MI, as early as school age.
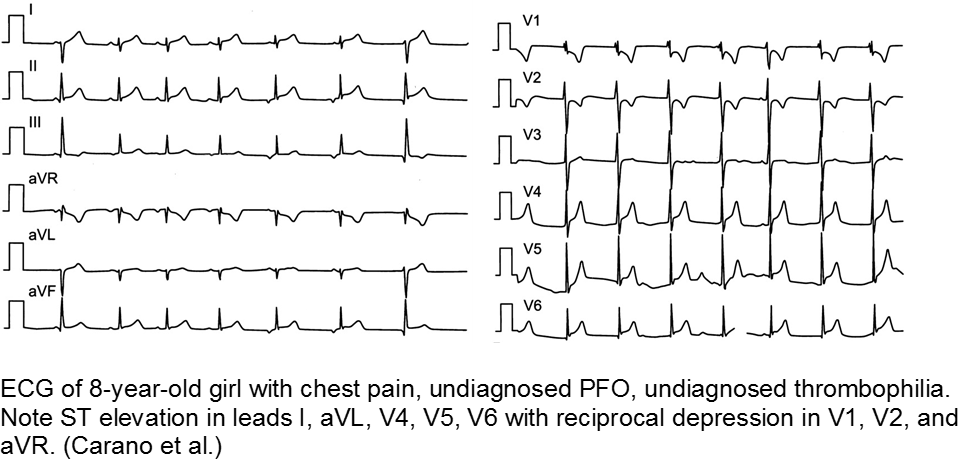
The previously well child now decompensated: undiagnosed thrombophilia
Asymptomatic patent foramen ovale (PFO) is the cause of some cases of cryptogenic vascular disease, such as stroke and MI. However, the presence of PFO alone does not connote higher risk. When paired with an inherited or acquired thrombogenic condition, the venous thrombus may travel from the right-sided circulation to the left, causing distal ischemia. Many of these cases are unknown until a complication arises.
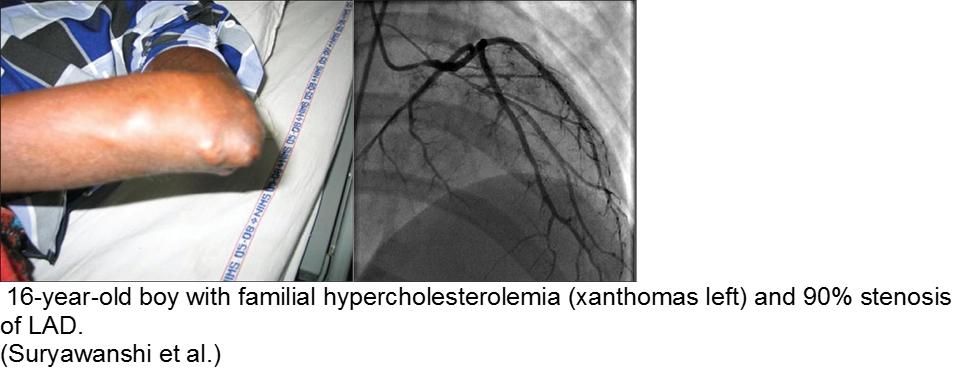
The chronically worried, now with a reason: hypercholesterolemia
A family history of adult-onset hypercholesterolemia is not necessarily a risk factor for early complications in children, provided the child does not have the same acquired risk factors as adults (e.g. obesity, sedentary lifestyle, smoking, etc). Parents may seek help in the ED for children with chest pain and no risk factors, but adult parents who have poor cholesterol profiles.
The exception is the child with familial hypercholesterolemia, who is at risk for accelerated atherosclerosis and MI.
Infectious
Myocarditis has varied etiologies, including infectious, medications (chemotherapy agents), immunologic (rheumatologic, transplant rejection), toxins (arsenic, carbon monoxide, heavy metals such as iron or copper), or physical stress (electrical injury, heat illness, radiation).
In children, the most common cause of myocarditis is infectious (viruses, protozoa, bacteria, fungal, parasites). Of these, viral causes are the most common (adenovirus, enterovirus, echovirus, rubella, HHV6).
The verbal child may complain of typical chest complaints, or may come in with flu-like illness and tachycardia or ill appearance out of proportion to presumed viral illness.
The most common presenting features in children with myocarditis are: shortness of breath, vomiting, poor feeding, hepatomegaly, respiratory distress, and fever.
The infant in shock after a ‘cold’: myocarditis
Beware of the poor feeding, tachycardic, ill appearing infant who “has a cold” because everyone else around him has a ‘cold’. That may very well be true, but any virus can be invasive with myocardial involvement. Infants are only able to increase their cardiac output through increasing their heart rate; they cannot respond to increased demands through ionotropy. Look for signs of acute heart failure, such as hepatomegaly, respiratory distress, and sacral edema.
The child with tachycardia out of proportion to complaint: myocarditis
The previously healthy child with “a bad flu” may simply be very symptomatic from influenza-like illness, or he may be developing myocarditis. Look for chest pain and tachycardia out of proportion to presumed illness, and constant chest pain, not just associated with cough.
The “pneumonia” with suspicious chest x-ray: myocarditis
Acute heart failure may mimic viral pneumonia. Look for disproportionate signs and symptoms.
Toxins
Younger children may get into others’ medications, be given dangerous home remedies, take drugs recreationally, have environmental exposures (heavy metals), suffer from a consequence of a comorbidity (iron or copper overload) or have adverse events from generally safe medications.
The hyperactive boy with a hyperactive precordium: methylphenidate
Attention deficit hyperactivity disorder (ADHD) is growing in rate of diagnosis and use of medications. As the only medical diagnosis based on self-reported criteria, many children are given stimulants regardless of actual neurologic disorder; with a higher proportion of children exposed to stimulants, adverse effects are seen more commonly.
Methylphenidate is related to amphetamine, and they both are dopaminergic drugs. Their mechanisms of action are different, however. Methylphenidate increases neuronal firing rate. Methamphetamine reduces neuronal firing rate; cardiovascular sequelae such as MI and CHF are more common in chronic methamphetamine use.
Although methylphenidate is typically well tolerated, risks include dysrhythmias such as ventricular tachycardia.
The child with seizure disorder and chest pain: anti-epileptics
Some anti-epileptic agents, such as carbamazepine, promote a poor lipid profile, leading to atherosclerosis and early MI. Case reports include school-aged children on carbamazepine who have foamy cells in the coronary arteries, aorta, and vasa vasorum on autopsy. It is unclear whether this is a strong association.
The spice trader: synthetic cannabinoids
Synthetic cannabinoids are notoriously difficult to regulate and study, as the manufacturers label them as “not for human consumption”. Once reports surface of abuse of a certain compound, the formula is altered slightly and repackaged, often in a colorful or mysterious way that is attractive to teenagers.
The misperceptions are: are a) synthetics are related to marijuana and therefore safe and b) marijuana is inherently “safe”. Both tend to steer unwitting teens to take these unknown entities. Some suffer MI as a result.
Exposure to tetrahydrocannabinol (THC) in high-potency marijuana has been linked to myocardial ischemia, ventricular tachycardia, and ventricular fibrillation. Marijuana can increase the heart rate from 20-100%, depending on the amount ingested.
K2 (“kush 2.0”) or Spice (Zohai, Genie, K3, Bliss, Nice, Black Mamba, fake weed, etc) is a mixture of plant leaves doused in synthetic chemicals, including cannabinoids and fertilizer (JWH-108), none of which are tested or safe for human consumption.
Synthetic cannabinoids have a higher affinity to cannabinoid receptors, conferring higher potency, and therefore worse adverse effects. They are thought to be 100 to 800 times more potent as marijuana.
Bath salts (Purple Wave, Zoom, Cloud Nine, etc) can be ingested, snorted, or injected. They typically include some form of cathinone, such as mephedrone, similar to the substance found in the naturally occurring khat plant. Hallucinations, palpitations, tachycardia, MI, and dysrhythmias have been reported from their use as a recreational drug.
Chest pain with marijuana, synthetic cannabinoid, or bath salt ingestion should be investigated and/or monitored.
Riding that train: high on cocaine
Cocaine is a well-known cause of acute MI in young people. In addition to the direct stimulant causes acutely, such as hypertension, tachycardia, and impaired judgement (coingestions, risky behavior), chronic cocaine use has long-term sequelae. Cocaine causes accelerated atherosclerosis. That, in conjunction with arterial vasospasm and platelet activation, is a recipe for acute MI in the young.
Cranky: methamphetamine
Methamphetamine is a highly addictive stimulant that is relatively inexpensive and widely available. Repeated use causes multiple psychiatric, personality, and neurologic changes. Risky behavior, violence, and motor vehicle accidents are all linked to this drug.
Like cocaine, methamphetamine may cause fatal dysrhythmias, acute MI from demand ischemia, and long-term sequelae such as congestive heart failure.
Summary
Acute MI is a challenging presentation in children:
- Easily missed: uncommon and atypical
- Varied etiology
- Respect vague symptoms with a non-reassuring H&P
- Try to detect it: CATH IT!
References
Congenital
AboulHosn JA et al. Fontan Operation and the Single Ventricle. Congenit Heart Dis. 2007; 2:2-11.
Aliku TO et al. A case of anomalous origin of the left coronary artery presenting with acute myocardial infarction and cardiovascular collapse. African Health Sci. 2014; 14(1): 23-227.
Andrews RE et al. Acute myocardial infarction as a cause of death in palliated hypoplastic left heart syndrome. Heart. 2004; 90:e17.
Canale LS et al. Surgical treatment of anomalous coronary artery arising from the pulmonary artery. Interactive Cardiovascaulr and Thoracic Surgery. 2009; 8:67-69.
Güvenç O et al. Correctable Cause of Dilated Cardiomyopathy in an Infant with Heart Failure: ALCAPA Syndrome. J Curr Pediatr. 2017; 15:47-50.
Hastings RS et al. Embolic Myocardial Infarction in a Patient with a Fontan Circulation. World Journal for Pediatric Congenital Heart Surgery. 2014; 5(4)L631-634.
Hoffman JIE et al. Electrocardiogram of Anomalous Left Coronary Artery From the Pulmonary Artery in Infants. Pediatr Cardiol. 2013; 34(3):489-491.
Kei et al. Rare Case of Myocardial Infarction in a 19-Year-Old Caused by a Paradoxical Coronary Artery Embolism. Perm J.2015; 19(2):e107-e109.
Liu Y, Miller BW. ALCAPA Presents in an Adult with Exercise Inlerance but Preserved Cardiac Function. Case Reports Cardiol. 2012; AID 471759.
Möhlenkamp S et al. Update on Myocardial Bridging.Circulation. 2002;106:2616-2622.
Murgan SJ et al. Acute myocardial infraction n the neonatal period. Cardiol Young. 2002; 12:411-413.
Sieweke JT et al. Myocardial infarction in grown up patients with congenital heart disease: an emergening high-risk combination. International Journal of Cardiology. 2016; 203:138-140.
Schwerzmann M et al. Anomalous Origin of the Left Coronary Artery From the Main Pulmonary Artery in Adults. Circulation. 2004; 110:e511-e513.
Tomkewicz-Pajak L et al. Arterial stiffness in adult patients after Fontan procedure. Cardiovasculr Ultrasound. 2014; 12:15.
Varghese MJ et al. The caveats in the diagnosis of anomalous origin of left coronary artery from pulmonary artery (ALCAPA). Images Paediatr Cardiol. 2010; 12(3): 3–8.
Autoimmune
Ayala et al. Acute Myocardial Infarction in a Child with Systemic Lupus Erythematosus and Antiphospholipid Syndrome. Turk J Rheumatol. 2009; 24:156-8.
Nakano H et al. Clinical characteristics of myocardial infarction following Kawasaki disease: Report of 11 cases. J Pediatr. 1986; 108(2):198-203.
Pongratz G et al. Myocardial infarction in an adult resulting from coronary aneurysms previously documented in childhood after an acute episode of Kawasaki’s disease. European Heart J. 1994. 15:1002-1004.
Newburger JW et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease. A Statement for Health Professionals From the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747-2771.
Son MB et al. Kawaski Disease. Pediatr Rev. 2013; 34(4).
Yuan S. Cardiac surgical procedures for the coronary sequelae of Kawasaki disease. Libyan J Med. 2012; 7:19796.
Trauma
Abdolrahim SA et al. Acute Myocardial Infarction Following Blunt Chest Trauma and Coronary Artery Dissection. J Clin Diagnost Res. 2016; 10(6):14-15.
Galiuto L et al. Post-traumatic myocardial infarction with hemorrhage and microvascular damage in a child with myocardial bridge: is coronary anatomy actor or bystander. Signa Vitae. 2013; 8(2):61-63.
Janella BL et al. Acute Myocardial Infarction related to Blunt Thoracic Trauma. Arq Bras Cardiol. 2006; 87:e168-e171.
Liu X et al. Acute myocardial infarction in a child with myocardial bridge World J Emerg Med. 2011; 2(1):70-72.
Long WA et al. Childhood Traumatic Infarction Causing Left Ventricular Aneurysm: Diagnosis by Two-Dimensional Echocardiography. JACC. 1985; 5(6):1478-83.
Smith S. Right Bundle Branch Block after Blunt Trauma: A Tragic Case. [Blog Post] July 22, 2012. Retrievable at: http://hqmeded-ecg.blogspot.com/2012/07/right-bundle-branch-block-after-blunt.html.
Hematologic
Carano N et al. Acute Myocardial Infarction in a Child: Possible Pathogenic Role of Patent Foramen Ovale Associated with Heritable Thrombophilia. Pediatr. 2004; 114(2):255-258.
Chacko P et al. Myocardial Infarction in Sickle Cell Disease. J Cardiovascl Transl Res. 2013; 6(5):752-761.
De Montalembert M et al. Myocardial ischaemia in children with sickle cell disease. Arch Dis Child. 2004; 89:359-362.
Gladwin MT et al. Cardiovascular Abnormalities in Sickle Cell Disease. JACC. 2012; 59(13):1123-1133.
Osula S et al. Acute myocardial infarction in young adults: causes and management. Postgrad Med J. 2002; 78:27-30.
Silva JMP et al. Premature acute myocardial infarction in a child with nephrotic syndrome. Pediatr Nephrol. 2002; 17:169-172.
Suryawanshi SP. Myocardial infarction in children: Two interesting cases. Ann Pediatr Cardiol. 2011 Jan-Jun; 4(1): 81–83.
Infectious
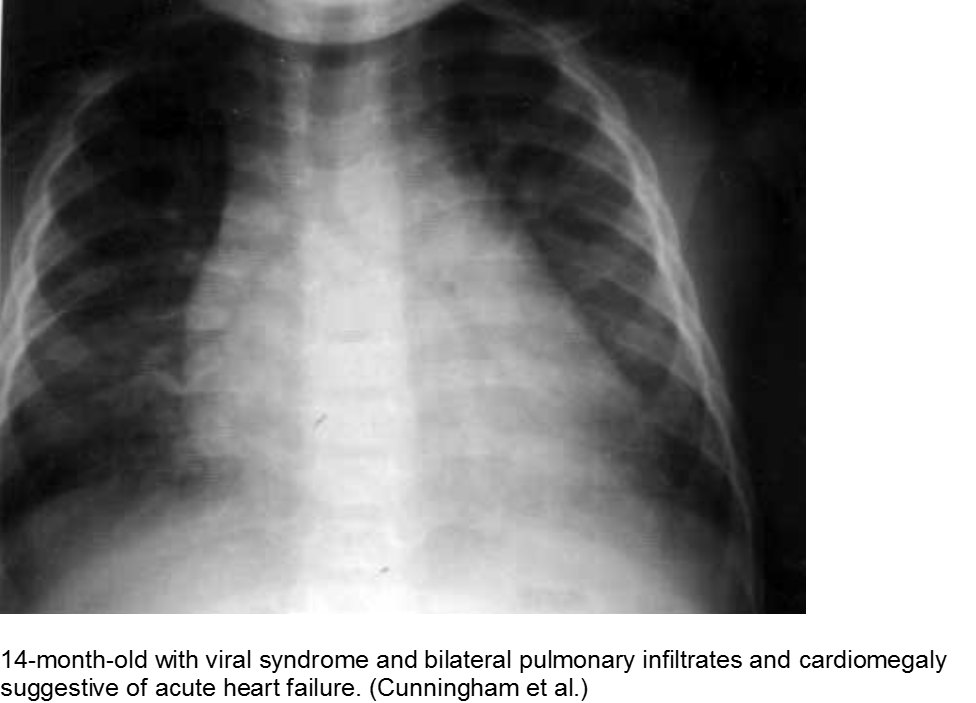
Cunningham R et al. Viral myocarditis Presenting with Seizure and Electrocardiographic Findings of Acute Myocardial Infarction in a 14-Month-Old Child. Ann Emerg Med. 2000; 35(6):618-622.
De Vettten L et al. Neonatal Myocardial Infarction or Myocarditis? Pediatr Cardiol. 2011; 32:492-497.
Durani Y et al. Pediatric myocarditis: presenting clinical characteristics. Am J Emerg Med. 2009; 27:942-947.
Erden I et al. Acute myocarditis mimicking acute myocardial infarction associated with pandemic 2009 (H1N1) influenza virus. Cardiol J. 2011; 552-555.
Hover MH et al. Acute Myocarditis Simulating Myocardial Infarction in a Child. Pediatr. 1191; 87(2):250-252.
Lachant D et al. Meningococcemia Presenting as a Myocardial Infarction. Case Reports in Critical Care. 2015; AID 953826.
Laissy JP et al. Differentating Myocardial Infarction from Myocarditis. Radiology. 2005; 237(1):75-82.
Miranda CH et al. Evaluation of Cardiac Involvement During Dengue Viral Infection. CID. 2013; 57:812-819.
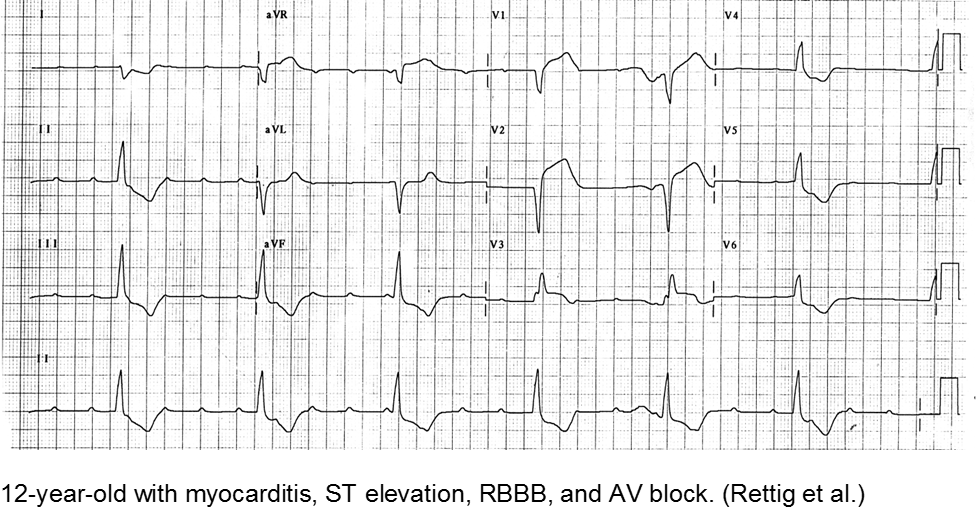
Rettig JS et al. Myocarditis in Children Requiring Critical Care Transport. In: "Diagnosis and Treatment of Myocarditis", Milei J, Ambrosio G (Eds). DOI: 10.5772/56177.
Toxins
De Chadarévian JP et al. Epilepsy, Atherosclerosis, Myocardial Infarction, and Carbamazepine. J Child Neurol. 2003; 18(2):150-151.
McIlroy G et al. Acute myocardial infarction, associated with the use of a synthetic adamantly-canabinoid: a case report. BMC Pharmacology and Toxicology. 2016; 17:2.
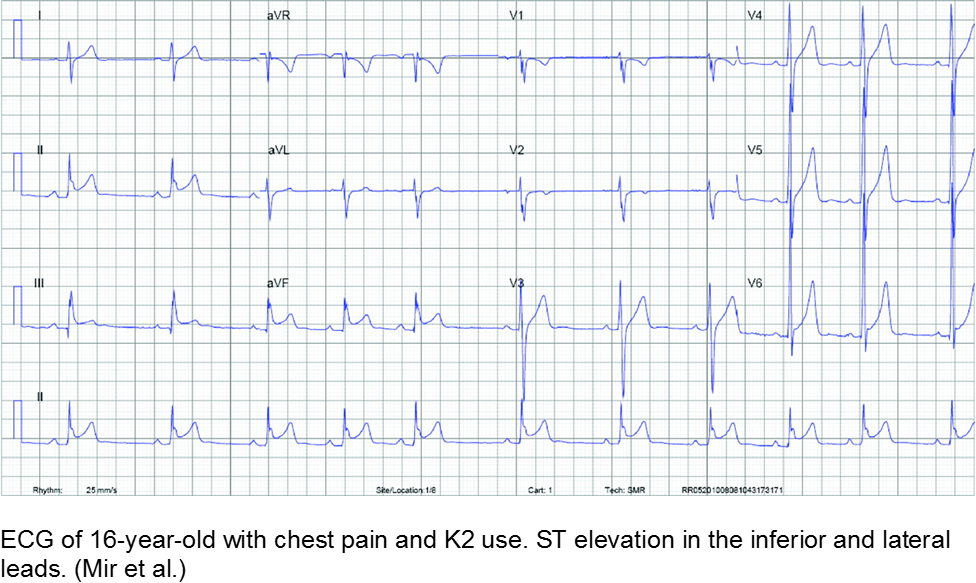
Mir A et al. Myocardial Infarction Associated with Use of the Synthetic Cannabinoid K2. Pediatr. 2011; 128(6):1-6
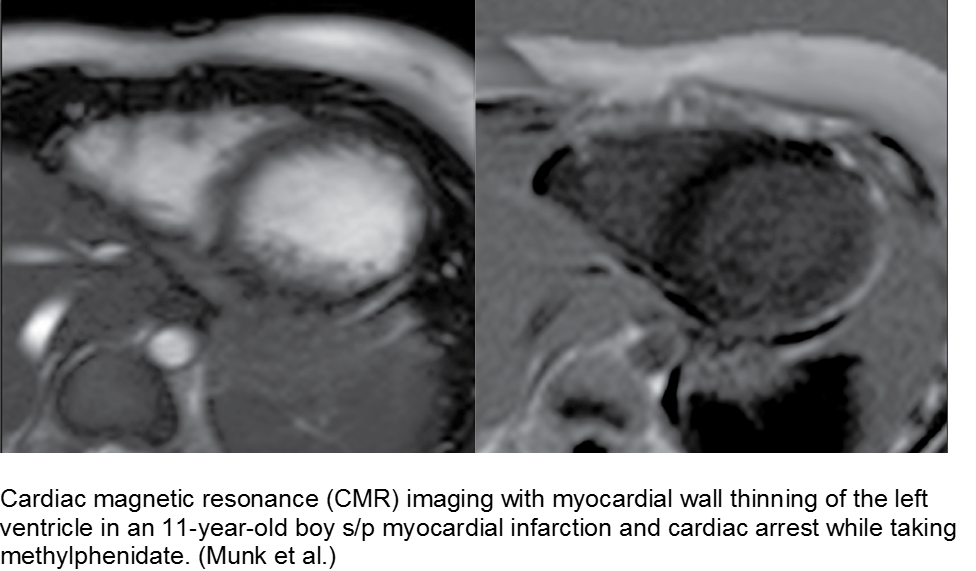
Munk K et al. Cardiac Arrest following a Myocardial Infarction in a Child Treated with Methylphenidate. Case Reports Pediatr. 2015; AID 905097.
Rezkalla SH et al. Cocaine-Induced Acte Mycardial Infarction. Clin Med Res. 2007; 5(3):172-176.
Schelleman H et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012 Feb;169(2):178-85.
Sheridan J et al. Injury associated with methamphetamine use: a review of the literature. Harm Reduction Journal, 2006; 3(14):1-18.
Stiefel G et al. Cardiovascular effects of methylphenidate, amphetamines and atomoxetine in the treatment of attention-deficit hyperactivity disorder. Drug Saf. 2010 Oct 1;33(10):821-42.
This post and podcast are dedicated to Edwin Leap, MD for his sanity and humanity in the practice of Emergency Medicine. Thank you, Dr Leap for all that you do.
Most newborns will have some jaundice. Most jaundice is benign.
So, how can we sort through the various presentations and keep our newborns safe?
Pathologic Jaundice
When a baby is born with jaundice, it’s always bad. This is pathologic jaundice, and it’s almost always caught before the baby goes home. Think about ABO-incompatbility, G6PD deficiency, Crigler-Najjar, metabolic disturbances, and infections to name a few. Newborns are typically screened and managed.
Physiologic Jaundice
Physiologic jaundice, on the other hand, is usually fine, until it’s not.
All babies have some inclination to develop jaundice. Their livers are immature. They may get a little dehydrated, especially if mother’s milk is late to come in. In today’s practice, we are challenged to catch those at risk for developing complications from rising bilirubin levels.
Hyperbilirubinemia is the result of at least one of three processes: you make too much, you don’t process it enough, or you don’t get rid of it fast enough.
Increased production
Bilirubin mostly comes from the recycling of red blood cells. Heme is broken down in in the liver and spleen to biliverdin then bilirubin.
Normal, full term babies without jaundice run a little high -- bilirubin production is two to three times higher than in adults, because they are born with a higher hematocrit. Also, fetal hemoglobin is great at holding on to oxygen, but has a shorter life span, and high turn-over rate, producing more bilirubin.
Impaired conjugation
Think of bilirubin as your email. Unconjugated bilirubin is your unread email. To process it or get rid of it – you have to open it. Of course, the more unread messages that accumulate, the more unwell you feel.
Conjugated bilirubin is your opened and processed email. So much easier to sort out, deal with, and get rid of.
Decreased excretion
Both unread email and unconjugated bilirubin continue to float around in your inbox. Unconjugated bilirubin keeps getting reabsorbed in the intestinal mucosa through enterohepatic circulation.
Processed email and conjugated bilirubin are easier to sort out. Conjugated bilirubin is water soluble, so it goes right into the read folder in your gallbladder, and is excreted off your inbox. Later on down the line in the intestine, conjugated bilirubin can’t be reabsorbed through the intestinal mucosa. Like when you open an email and forget about it – it passes on through, out of your system.
Newborns are terrible at answering emails. There is a lot of unread unconjugated bilirubin is floating around. The liver and spleen are just not able to keep up.
Also, newborns have a double-whammy administrative load. Normally, bacteria in the gut can further break down conjugated bilirubin to urobilin and get excreted in the urine. The infant’s gut is relatively sterile, so no admin assistance there. Just to add to the workload a poor little newborn has to do – he is being sabotaged by extra beta-glucuronidase which will take his hard-earned conjugated bilirubin and unconjugate it again, then recycle it, just like email you “mark as unread”.
How Does this All Go Down?
The recommended followup is 48 hours after discharge from the nursery for a routine bilirubin check, often in clinic, and often via the transcutaneous route.
More Specifically:
| Infant Discharged | Should Be Seen by Age |
| Before age 24 h | 72 h |
| Between 24 and 48 h | 96 h |
| Between 48 and 72 h | 120 h |
The neonate will end up in your ED off hours, if there is concern, if his status deteriorates, or simply by chance. We need to know how to manage this presentation, because time is of the essence to avoid complications if hyperbilirubinemia is present.
Critical Action #1:
Assess risk for developing severe hyperbilirubinemia.
This will tell you: check now in ED or defer to clinic (default is to check).
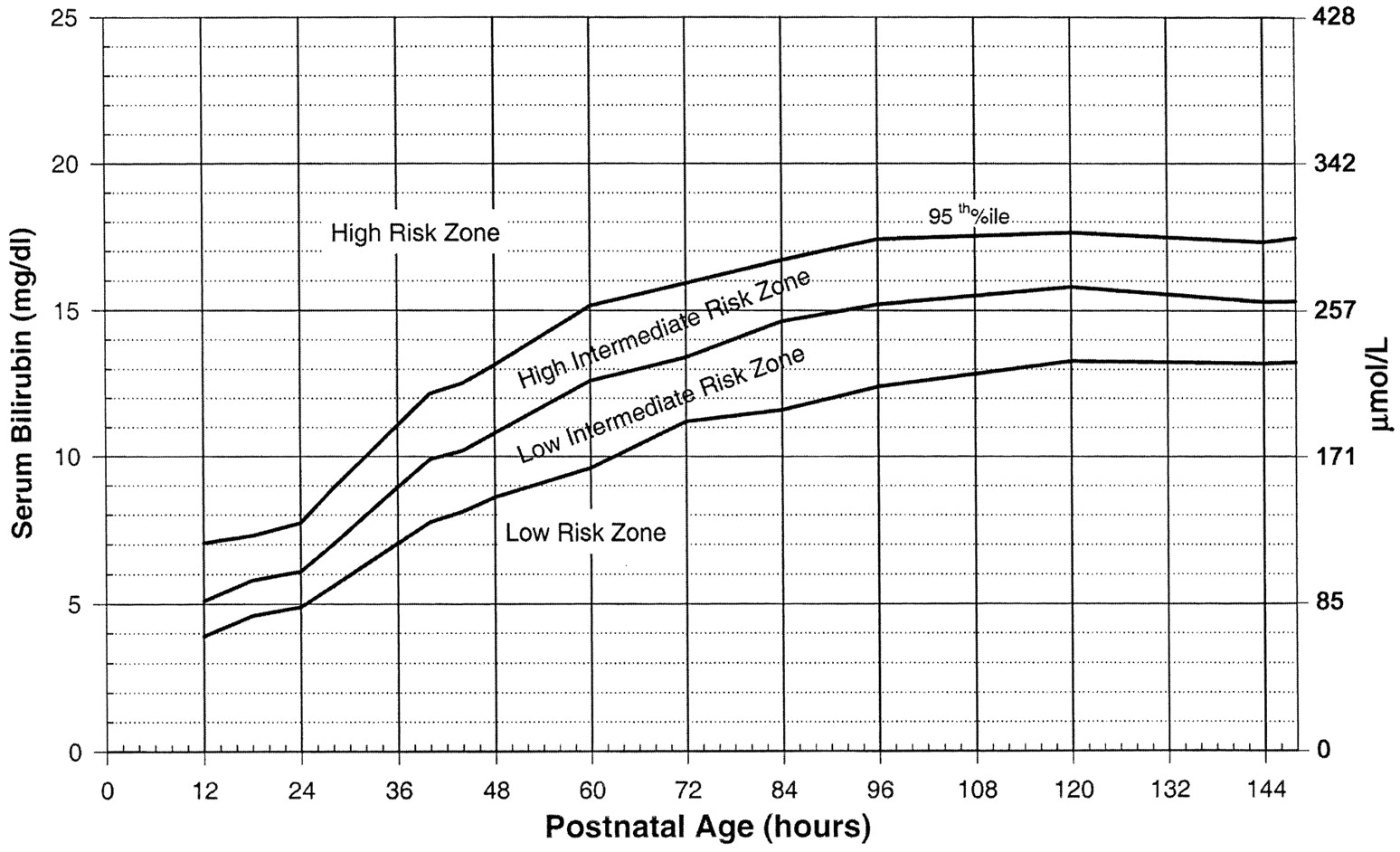
| Risk Factors for Developing Hyperbilirubinemia |
| Total serum bilirubin/Transcutaneous bilirubin in high-risk zone |
| Jaundice in first 24 hours |
| ABO incompatibility with positive direct Coombs, known hemolytic disease, or elevated ETCO |
| Gestational age 35-36 weeks |
| Prior sibling had phototherapy |
| Cephalohematoma or bruising |
| Exclusive breastfeeding, especially with poor feeding or weight loss |
| East Asian Race |
Critical Action #2
Check bilirubin and match this with how old the child is -- in hours of life -- at the time of bilirubin measurement.
This will tell you: home or admission.
Use the Bilitool or Bhutani Nomogram (below).
Can I go Home Now?
In general, babies at low-risk and low-intermediate risk can go home (see below). Babies at high-intermediate or high risk are admitted (see below).
Critical Action #3:
Assess risk for developing subsequent neurotoxicity.
This will tell you: a) phototherapy or b) exchange transfusion
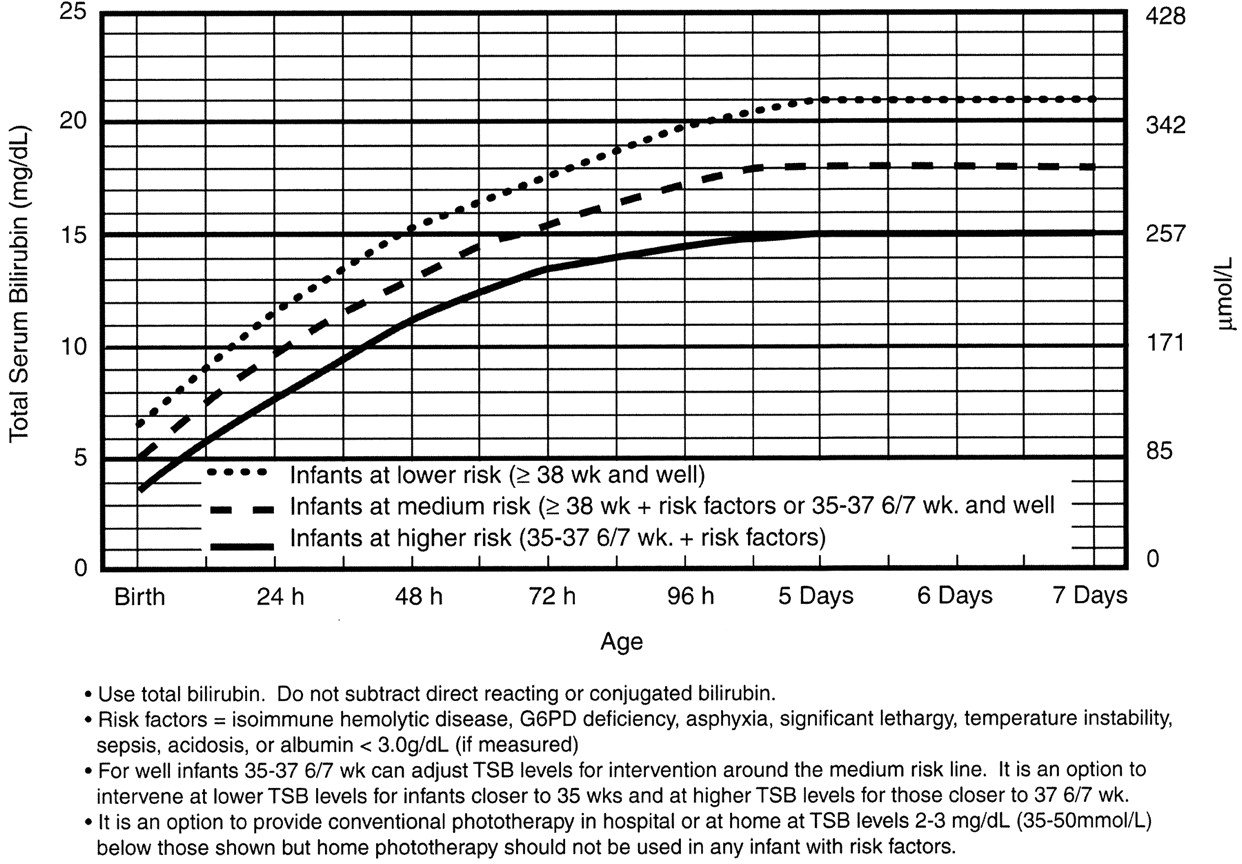
Phototherapy Now?
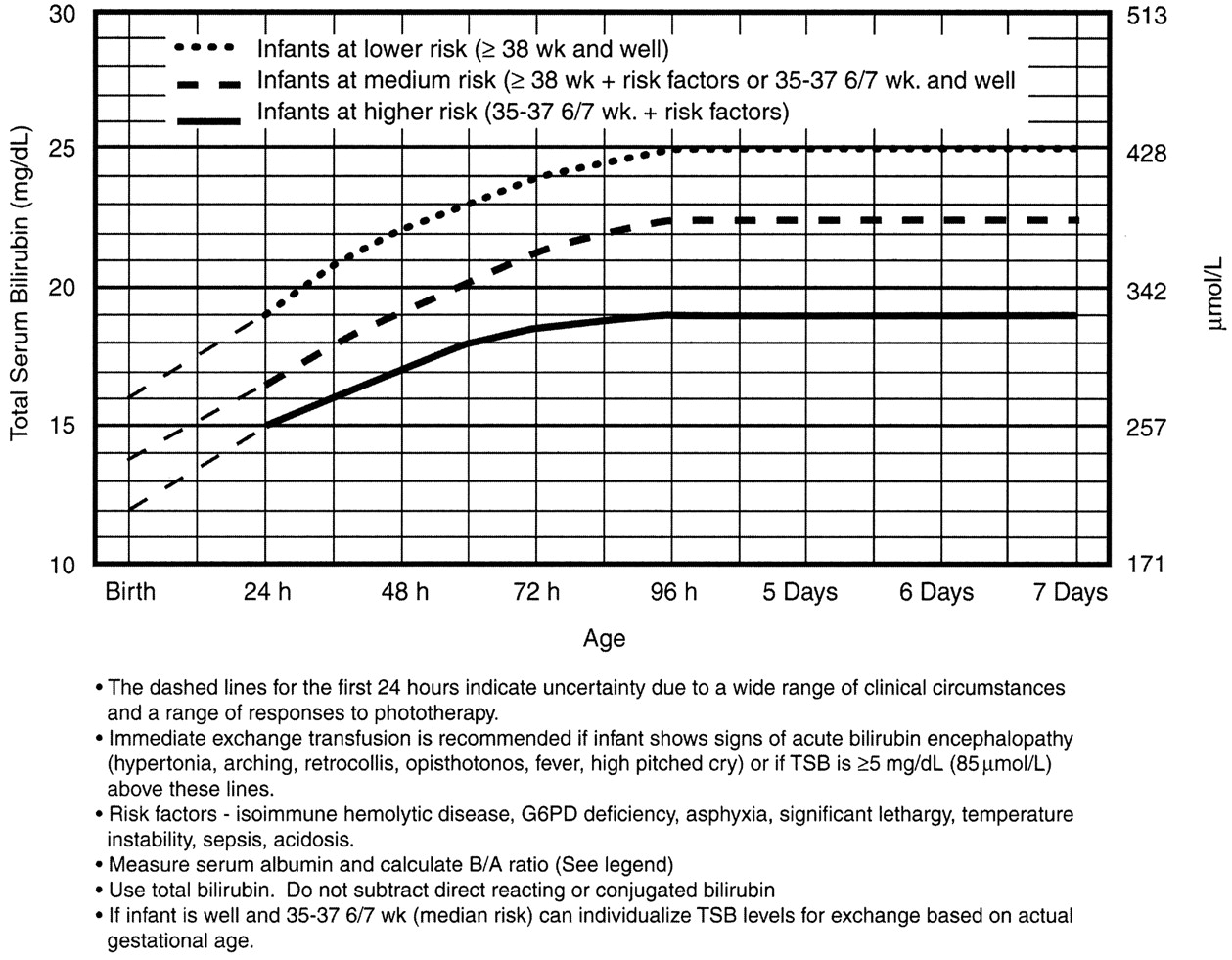
Exchange Transfusion Now?
Home care
The neonate who is safe to go home is well appearing, and not dehydrated. His total bilirubin is in the low to low-intermediate risk for developing severe hyperbilirubinemia, and he is not at high risk for neurotoxicity based on risk factors.
Babies need to stay hydrated. Breast feeding mothers need encouragement and need to offer feeds 8-12 times/day – an exhausting regimen. The main message is: stick with it. Make sure to enlist the family's help and support to keep Mom hydrated, eating well, and resting whenever she can. Supplementing with formula or expressed breast milk is not routinely needed. Be explicit that the neonate should not receive water or sugar water – it can cause dangerous hyponatremia. A moment of solid precautionary advice could avert a disaster in the making.
The child’s pediatrician will help more with this, and you can remind nursing mothers of the excellent La Leche League – an international group for breastfeeding support. They have local groups everywhere, including a hotline to call.
Nursery Care
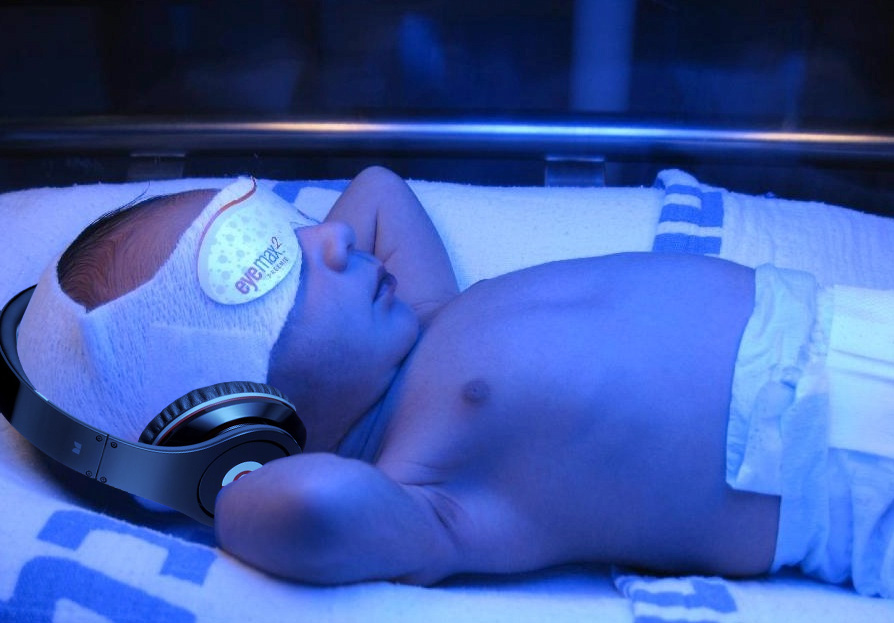
If the baby is at high intermediate or high risk for hyperbilirubinemia, then he should be admitted for hydration, often IV. Most babies with hyperbilirubinemia are dehydrated, which just exacerbates the problem.
Bililights or biliblankets, provide the baby with the right blue spectrum of light to isomerize bilirubin to the more soluble form. Traditionally, we have thought them to be more effective or safer than filtered sunlight. A recent randomized control trial by Slusher et al. in the New England Journal of Medicine compared filtered sunlight versus conventional phototherapy for safety and efficacy in a resource-poor environment. These were all term babies with clinically significant jaundice in Nigeria. To standardize the intervention, they used commercial phototherapy canopies that remove most UV rays. None of them became dehydrated or became sunburned. The filtered sunlight resulted in a 93% successful treatment versus 90% for conventional phototherapy. My take away: we now have some evidence basis for using filtered sunlight as an adjunct for babies well enough to go home.
Critical Care
Although rare, the critically ill neonate with hyperbilirubinemia requires immediate intervention.
He will be dehydrated – possibly in shock. He will be irritable.
Or, he may just have a dangerously high bilirubin level – at any minute he could develop bilirubin induced neurologic dysfunction, or BIND, especially when bilirubin concentrations reach or surpass 25 mg/dL (428 micromol/L). The bilirubin is so concentrated that it leeches past the blood brain barrier and causes neuronal apoptosis. BIND is a spectrum from acute bilirubin encephalopathy to kernicterus, all involving some disorder in vision, hearing, and later gait, speech, and cognition.
Acute bilirubin encephalopathy starts subtly. The neonate may be sleepy but hypotonic or have a high-pitched cry; he maybe irritable or inconsolable, jittery or lethergic.
The dehydration and neurologic dysfnction from the hyperbilirubinemia may even cause fever. Check the bilirubin in any neonate you are working up for sepsis.
Acute bilirubin encephalopathy may progress to an abnormal neurologic exam, seizures, apnea, or coma.
Kernicterus is the final, permanent result of bilirubin encephalpathy. The child may have choreoathetoid cerebral palsy with chorea, tremor, ballismus, and dystonia. He may have sensorineural hearting loss, or cognitive dysfunction.
It is for this reason that any child sick enough to be admitted should be considered for exchange transfusion. Most babies need just a little gentle rehydration and bililights, but to be sure, the admitting team will look at a separate nomogram to gage the child’s risk and decide whether to pull the trigger on exchange transfusion. For our purposes, a ballpark estimate is that if the total serum bilirubin is 5 mg/dL above the phototherapy threshold, or if they have any red flag signs or symptoms, then exchange transfusion should be started.
Exchange transfusion involves taking small aliquots of blood from the baby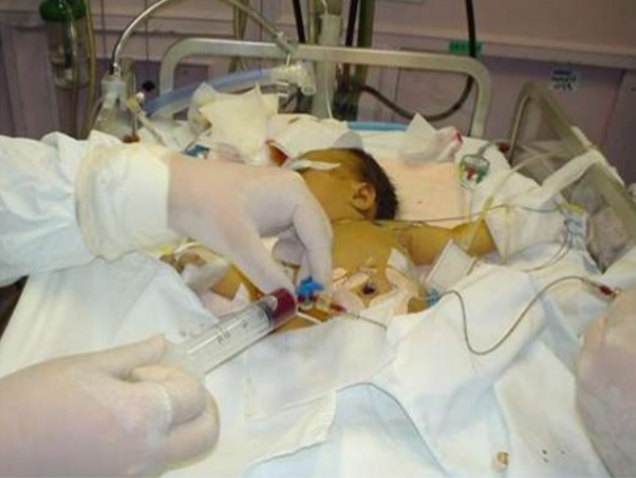 and replacing them with donor blood. It’s often a manual procedure, done with careful monitoring. It can be done with any combination of umbilical arteries or veins with peripheral arteries or veins. In general, arteries are the output, veins are for transfusion. The baby may need a double-volume exchange, which ends up replacing about 85% of circulating blood, a single-voume exchange, replacing about 60% of blood, or any fraction of that with apartial volume exchange. It is a very delicate procedure that requires multiple hours and often multiple staff.
and replacing them with donor blood. It’s often a manual procedure, done with careful monitoring. It can be done with any combination of umbilical arteries or veins with peripheral arteries or veins. In general, arteries are the output, veins are for transfusion. The baby may need a double-volume exchange, which ends up replacing about 85% of circulating blood, a single-voume exchange, replacing about 60% of blood, or any fraction of that with apartial volume exchange. It is a very delicate procedure that requires multiple hours and often multiple staff.
For our pruposes, just be aware that the jaundiced baby in front of you may need escalation of his care.
Summary
Find out the hour of life of the baby at the time of bilirubin measurement. Identify risk factors for developing severe hyperbilirubinemia and/or neurotoxicity
The child with low to low-intermediate risk may be a good outpatient candidate provided he is well, not dehydrated, and follow-up is assured.
The child with high-intermediate to high-risk for developing severe hyperbilirubinemia should be admitted for hydration, bililights, and/or assessment for exchange transfusion.
The unwell child with or without current neurologic findings should have immediate exchange transfusion.
References
Benitz WE. Hospital Stay for Healthy Term Newborn Infants. Pediatrics. 2015; 135(5):948-53.
Lauer BJ, Spector ND. Hyperbilirubinemia in the Newborn. Pediatrics in Review. 2011; 32(8):341-9.
This post and podcast are dedicated to Gita Pensa, MD, for her commitment to #FOAMed and passion for asynchronous learning and education innovation.
Children the world over are fascinated with what can possibly “fit” in their orifices. Diagnosis is often delayed. Anxiety abounds before and during evaluation and management.
Most common objects:1,2
| Food | Coins | Toys |
| Insects | Balls, marbles | Balloons |
| Magnets | Crayon | Hair accessories, bows |
| Beads | Pebbles | Erasers |
| Pen/marker caps | Button batteries | Plastic bags, packaging |
Non-pharmacologic techniques
Set the scene and control the environment. Limit the number of people in the room, the noise level, and minimize “cross-talk”. The focus should be on engaging, calming, and distracting the child.
Quiet room; calm parent; “burrito wrap”; guided imagery; have a willing parent restrain the child in his or her lap – an assistant can further restrain the head.
Procedural Sedation
Most foreign bodies in the ear, nose, and throat in children can be managed with non-pharmacologic techniques, topical aids, gentle patient protective restraint, and a quick hand. Consider sedation in children with special health care needs who may not be able to cooperate and technically delicate extractions. Ketamine is an excellent agent, as airway reflexes are maintained.3 Remember to plan, think ahead: where could the foreign body may be displaced if something goes wrong? You may have taken away his protective gag reflex with sedation. Position the child accordingly to prevent precipitous foreign body aspiration or occlusion.
L’OREILLE – DAS OHR – вухо – THE EAR – LA OREJA – 耳 – L'ORECCHIO
Essential anatomy: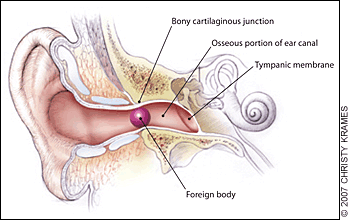
The external auditory canal. Foreign bodies may become lodged in the narrowing at the bony cartilaginous junction.4 The lateral 1/3 of the canal is flexible, while the medial 2/3 is fixed in the temporal bone – here is where many foreign bodies are lodged and/or where the clinician may find evidence of trauma.
Pearls:
- Ask yourself: is it graspable or non-graspable?5
- Graspable: 64% success rate, 14% complication rate
- Non-graspable: 45% success rate, 70% complication rate5
- If there is an insect in the external auditory canal, kill it first. They will fight for their lives if you try to dismember or take them out. “In the heat of battle, the patient can become terrorized by the noise and pain and the instrument that you are using is likely to damage the ear canal.”5,6 Use lidocaine jelly (preferred), viscous lidocaine (2%), lidocaine solution (2 or 4%), isopropyl alcohol, or mineral oil.
- Vegetable matter? Don’t irrigate it – the organic material will swell against the fixed structure, and cause more pain, make it much harder to extract, and may increase the risk of infection.
Pifalls:
- Failure to inspect after removal – is there something else in there?
- Failure to assess for abrasions, trauma, infection – if any break in skin, give prophylactic antibiotic ear drops
- Law of diminishing returns: probability of successful removal of ear foreign bodies declines dramatically after the first attempt
LE NEZ – DIE NASE – ніс – THE NOSE – LA NARIZ – 鼻 – IL NASO
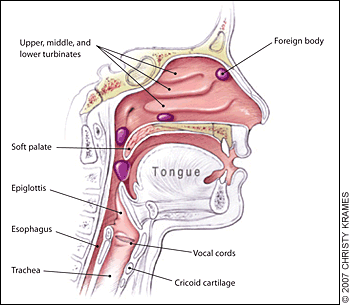 Essential anatomy:
Essential anatomy:
Nasopharyngeal and tracheal anatomy. Highlighted areas indicate points at which nasal foreign bodies may become lodged.4
Pearls:
- Consider using topical analgesics and vasoconstrictors to reduce pain and swelling – and improve tolerance of/cooperation with the procedure. Use 0.5% oxymetolazone (Afrin) spray and a few drops of 2 or 4% Pros: as above. Cons: possible posterior displacement of the foreign body.7
- Be ready for the precipitous development of an airway foreign body
Pitfalls:
- Beware of unilateral nasal discharge in a child – strongly consider retained foreign body.8
- Do not push a foreign body down the back of a patient's throat, where it may be aspirated into the trachea.
- Be sure to inspect the palate for “vacuum effect”: small or flexible objects may be found on the roof of the mouth, just waiting to be aspirated.
LA GORGE – DER HALS – горло – THE THROAT – LA GARGANTA – 喉 – LA GOLA
Before we go further –
Remember that a foreign body in the mouth or throat can precipitously become a foreign body in the airway. Foreign body inhalation is the most common cause of accidental death in children less than one year of age.9,10
Go to BLS maneuvers if the child decompensates.
Infants under 1 year of age – back blows: head-down, 5 back-blows (between scapulae), 5 chest-thrusts (sternum). Reassess, repeat as needed.
Children 1 year and up, conscious – Heimlich maneuver: stand behind patient with arms positioned under the patient’s axilla and encircling the chest. The thumb side of one fist should be placed on the abdomen below the xiphoid process. The other hand should be placed over the fist, and 5 upward-inward thrusts should be performed. This maneuver should be repeated if the airway remains obstructed. Alternatively, if patient is supine, open the airway, and if the object is readily visible, remove it. Abdominal thrusts: place the heel of one hand below the xiphoid, interlace fingers, and use sharp, forceful thrusts to dislodge. Be ready to perform CPR.
Children 1 year and up, unconscious – CPR: start CPR with chest compressions (do not perform a pulse check). After 30 chest compressions, open the airway. If you see a foreign body, remove it but do not perform blind finger sweeps because they may push obstructing objects further into the pharynx and may damage the oropharynx. Attempt to give 2 breaths and continue with cycles of chest compressions and ventilations until the object is expelled.
Chest films are limited: 80% of airway foreign bodies are radiolucent.11 Look for unilateral hyperinflation on expiratory films: air trapping.
Essential anatomy:
Most esophageal foreign bodies in children occur at the level of the thoracic inlet / cricopharyngeus muscle (upper esophageal sphincter). Other anatomically narrow sites include the level of the aortic arch and the lower esophageal sphincter.
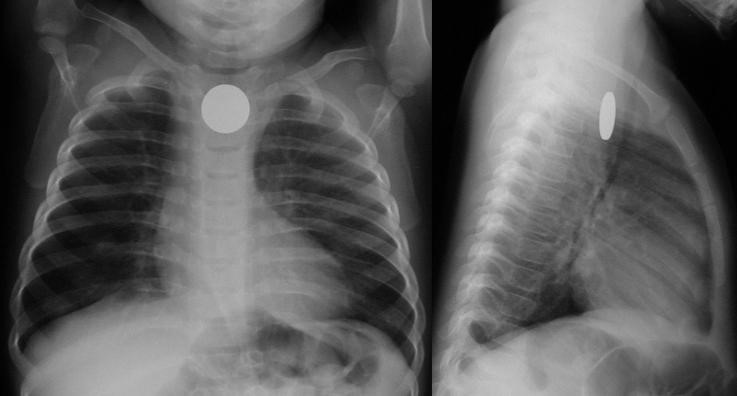
Coin en face – in the esophagus – lodged at the thoracic inlet.12 The pliable esophagus accommodates the flat coin against the flat aspect of the vertebra.11
Beware the “double-ring” sign: this is a button battery13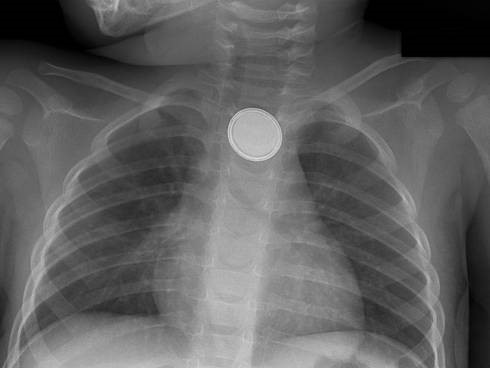
This is an emergency: the electrolyte-rich mucosa conducts a focal current from the narrow negative terminal of the battery, rapidly causing burn, necrosis, and possibly perforation. Emergent removal is required.
Button batteries that have passed into the stomach do not require emergent intervention – they can be followed closely.
Not a button battery, not a sharp object, not a long object?
If there is no obstruction, consider revaluation the next day – may wait up to 24 hours for passage.14 Sharieff et al.15 found that coins found in the mid to distal esophagus within 24 hours all passed successfully.
What conditions prompt urgent removal?
Size
Infants: objects smaller than 2 cm wide and 3 cm long will likely pass the pylorus and ileocecal valve10
Children and adults: objects smaller than 2 cm wide and 5 cm long will likely pass the pylorus and ileocecal valve9
Character
Sharp objects have a high rate of perforation (35%)1
Pearls:
- History is essential. Believe the parents and assume there is an aspirated/ingested foreign body until proven otherwise.
- History of choking, has persistent symptoms and/or abnormal xray? Broncoscopy! Cohen et al.16 found that of 142 patients evaluated at a single site university hospital, 61 had a foreign body. Of the 61 patients, 42 had abnormal physical exams and radiographs and 17 had either abnormal physical exams or radiographs, and 2 had normal physical exams and radiographs, but both had a history of persistent cough. Bottom line: history of choking PLUS abnormal exam, abnormal films, or persistent symptoms, evaluate with bronchoscopy.
- For patients with some residual suspicion of an aspirated foreign body (mild initial or improving symptoms; possibly abnormal chest x-ray; low but finite risk), consider chest CT with virtual bronchoscopy as a rule-out strategy.17,18
- Outpatients who have passed a small and non-concerning object into the stomach or beyond: serial exams and observing stools – polyethylene glycol 3350 (MiraLAX) may be given for delayed passage19
Pifalls:
- A single household magnet will likely pass through the GI tract, with the aforementioned dimensional caveats. Two or more magnets, however, run the risk of attraction and trans-bowel wall pressure necrosis.
- Not all magnets are created equal. Neodymium magnet toys (“buckyballs”) were recalled in 2014 (but are still out there!) due to their highly attractive nature. These magnets must be removed to avoid bowel wall ischemia. 19–21
- Patients should avoid wearing belt buckles or metallic buttons in case of single magnet ingestion while waiting for the single magnet to pass

DES OUTILS DU MÉTIER – DIE HANDWERKSZEUG – Знаряддя праці
– TOOLS OF THE TRADE –
LAS HERRAMIENTAS DEL OFICIO – GLI ATTREZZI DEL MESTIERE – 仕事のツール
It’s best to keep your armamentarium as large as you can.
Curette
A small foreign body in the lateral 1/3 of the auditory canal may be amenable to a simple curettage. Hair beads (if the central hole is accessible) may be manipulated out with the angled tip of a rigid curette. Steady the operating hand by placing your hypothenar eminence on the child’s zygoma or temporal scalp, to avoid jutting the instrument into the ear canal with sudden movement. There is a large selection of disposable simple and lighted curettes on the market.
Right-angle Hook
Various eponymous hooks are available for this purpose; one in popular use is the Day hook, which may be passed behind the foreign body.22 An inexpensive and convenient alternative to the commercially available right-hooks is a home-made version: make your own by straightening out a paperclip and bending it to a right angle23 at 2-3 mm from the end (be sure not to use the type that have a friable shiny metallic finish, as the residue may be left behind in the ear canal). If it is completely lodged, use of a right-angle hook will likely only cause trauma to the canal.
Alligator forceps
Alligator forceps are best for grasping soft objects like cotton or paper. Smooth, round or oval objects are not amenable to extraction with alligator forceps. When using them, be sure to get a firm, central grip on the object, to avoid tearing it into smaller, less manageable pieces.
Pro tip: Look before you grip! Be careful to visualize the area you are gripping, to avoid pulling on (and subsequently avulsing) soft tissue in the ear canal.
Cyanoacrylate (Dermabond®, SurgiSeal®)
Apply cyanoacrylate to either side of a long wooden cotton swab (the lecturer prefers the cotton tip side, for improved grip/molding around object). Immediately apply the treated side to the object in the ear canal in a restrained patient. Steady the hypothenar eminence on the child’s face to avoid dislodgement of the cotton swab with sudden movement. Apply the treated swab to the foreign body for 30-60 seconds, to allow bonding. Slowly pull out the foreign body. Re-visualize the ear canal for other retained foreign bodies and abrasion or ear canal trauma.
Did the cyanoacrylate drip? Did the swab stick to the ear canal?
No problem – use 3% hydrogen peroxide or acetone.24 Pour in a sufficient amount, allow to work for 10 minutes. Both agents help to dissolve ear wax, the compound, or both. Repeat if needed to debond the cyanoacrylate from the ear canal.24,25
Irrigation
Irrigation is the default for non-organic foreign bodies that are not amenable to other extraction techniques. Sometimes the object is encased in cerumen, and the only “instrument” that will fit behind the foreign body is the slowly growing trickle of water that builds enough pressure to expulse it. Do not use if there is any organic material involved: the object will swell, causing much more pain, difficulty in extraction, and possibly setting up conditions for infection.
Position the child either prone or supine, gently restrain (as above). Let gravity work for you: don’t irrigate in decubitus position with the affected ear up. It may be more accessible to you, but you may never get the foreign body out.
To use a butterfly needle: use a small gage (22 or 24 g) butterfly set up, cut off the needle, connect the tubing to a 30 mL syringe filled with warm or room-temperature water. Insert the free end of the tubing gently, and “secure” the tubing with your pinched fingers while irrigating (think of holding an ETT in place just after intubation and before taping/securing the tube). Gently and slowly increase the pressure you exert as you irrigate.
To use an IV or angiocatheter: use a moderate size (18 or 20 g) IV, remove the needle and attach the plastic catheter to a 20 mL syringe, and irrigate as above.
Acetone
Acetone has been used successfully to remove chewing gum, Styrofoam, and superglue from the ear canal24,26,27 Use in cases where there is no suspicion of perforation of the tympanic membrane.
Docusate Sodium (Colace®)
Cerumen is composed of sebaceous ad ceruminous secretions and desquamated skin. Genetic, environmental, and anatomical factors combine to trap a foreign body in the external canal. Use of a ceruminolytic such as docusate sodium may help to loosen and liberate the foreign body.28 Caveat medicus: Adding docusate sodium will make the object more slippery – this may or may not be an issue given the nature of the foreign body.
In the case where loosening the ear wax may aid extraction (and will not cause a slippery mess), consider filling the ear canal will docusate sodium (Colace), having the child lie with the affected side up, waiting 15 minutes, and proceeding. This is especially helpful when planning for irrigation.
Magnets
Rare earth magnets (a misnomer, as their components are actually abundant) such as neodymium can be useful in retrieving metallic foreign bodies (e.g. button batteries in the nose or ears).29,30 Magnetic “pick-up tools” – used by mechanics, engineers, and do-it-yourselfers – are inexpensive and readily available in various sizes, shapes, and styles such as a telescoping extender. Look for a small tip diameter (to fit in the ear canal as well as the nose) and a strong “hold” (at least a 3-lb hold).
Alternatively, you may purchase a strong neodymium bar magnet (30- to 50-lb hold) to attach to an instrument such as an alligator forceps, pick-up forceps, or small hemostat (a pacemaker magnet may also work). The magnetic bar, placed in your palm at the base of the instrument, will conduct the charge (depending on the instrument) and allow you to retrieve many metallic objects.31 Although stainless steel is often said to be “non-magnetic”, it depends on the alloy used, and some may actually respond to the strong rare earth magnet. Most stainless steel has a minimum of 10.5% chromium, which gives the steel its 'stainless' properties (essentially corrosion resistance). A basic stainless steel has a “ferritic” structure and is magnetic. Higher-end stainless steel such as in kitchen cutlery have an “austenitic” structure, with more chromium added, and so less magnetic quality. (Ironically, the more “economical” instruments in the typical ED suture kit have less chromium, and so are more magnetic – use these with your neodymium bar magnet to conduct the magnetic charge and extract the metallic foreign body.)
Bottom line: if it’s metal, it’s worth a try to use a magnet. Even if the metal is very weakly magnetic, the strong neodymium magnet may still be able to exert a pull on it and aid in extraction.
Snare Technique
A relatively new method, described by Fundakowski et al.32 consists of using a small length of 24-gauge (or similar) wire (available inexpensively online, and kept in your personal “toolkit”; see Appendix B below) to make a loop that is secured by a hemostat (the 24-gauge wire is easily cut into strips with standard trauma scissors). After treatment with oxymetolazone (0.05%) and lidocaine (1 or 2%) topically, the loop is passed behind the foreign body (in the case report, a button battery). Pull the loop toward you until you feel that it is sitting up against the button battery. Now, turn the hemostat 90° to improve your purchase on the foreign body. Pull gently out. This technique is especially useful when the foreign body has created marked edema, either creating a vacuum effect or making it difficult for other instruments to pass.
Balloon Catheters (Katz extractor®, Fogarty embolectomy catheter)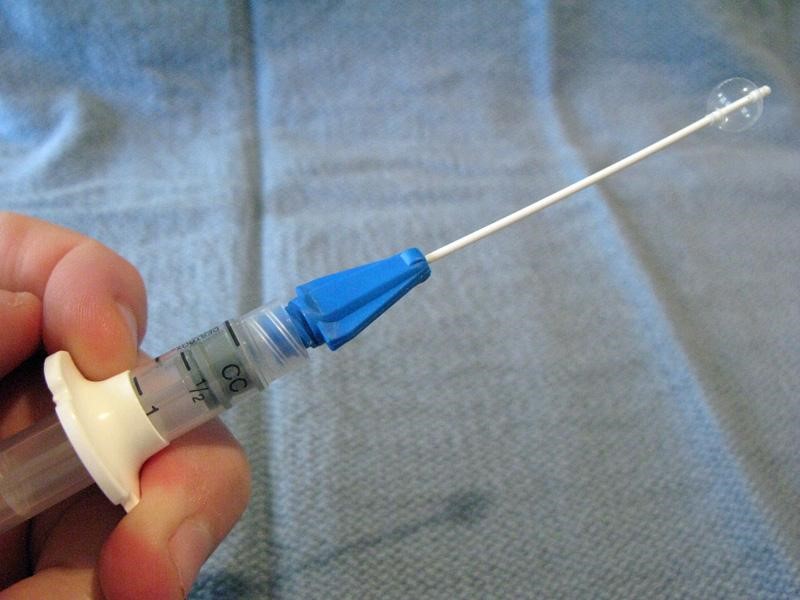
Small-caliber devices (5, 6, or 8 F) originally designed for use with intravascular or bladder catheterization may be used to extract foreign bodies from the ear or nose.7,33 A catheter designed specifically for foreign body use is the Katz extractor. Inspect the ear or nose for potential trauma and to anticipate bleeding after manipulation (especially the nose). Deflate the catheter and apply surgical lubricant or 2% lidocaine jelly. Insert the deflated catheter and gently pass the device past the foreign body. Inflate the balloon and slowly pull the balloon and foreign body out. Re-inspect after extraction.
NB, from the manufacturer of the Katz extractor, InHealth: “the Katz Extractor oto-rhino foreign body remover is intended principally for extraction of impacted foreign bodies in the nasal passages. This device may also be used in the external ear canal, based upon clinical judgment.”
Mother’s kiss
 The mother’s kiss was first described in 1965 by Vladimir Ctibor, a general practitioner from New Jersey.34 “The mother, or other trusted adult, places her mouth over the child’s open mouth, forming a firm seal as if about to perform mouth-to-mouth resuscitation. While occluding the unaffected nostril with a finger, the adult then blows until feeling resistance caused by closure of the child’s glottis, at which point the adult gives a sharp exhalation to deliver a short puff of air into the child’s mouth. This puff of air passes through the nasopharynx, out through the non-occluded nostril and, if successful, results in the expulsion of the foreign body. The procedure is fully explained to the adult before starting, and the child is told that the parent will give him or her a “big kiss” so that minimal distress is caused to the child. The procedure can be repeated a number of times if not initially successful.”34
The mother’s kiss was first described in 1965 by Vladimir Ctibor, a general practitioner from New Jersey.34 “The mother, or other trusted adult, places her mouth over the child’s open mouth, forming a firm seal as if about to perform mouth-to-mouth resuscitation. While occluding the unaffected nostril with a finger, the adult then blows until feeling resistance caused by closure of the child’s glottis, at which point the adult gives a sharp exhalation to deliver a short puff of air into the child’s mouth. This puff of air passes through the nasopharynx, out through the non-occluded nostril and, if successful, results in the expulsion of the foreign body. The procedure is fully explained to the adult before starting, and the child is told that the parent will give him or her a “big kiss” so that minimal distress is caused to the child. The procedure can be repeated a number of times if not initially successful.”34
Positive Pressure Ventilation with Bag Valve Mask
 This technique is an approximation of the above mother’s kiss technique – useful for unwilling parents or unsuccessful tries.10,25 The author prefers to position the child sitting up. A self-inflating bag-mask device is fitted with a very small mask: use an abnormally small mask (otherwise inappropriately small for usual resuscitative bag-mask ventilation) to fit over the mouth only. Choose an infant mask that will cover the child’s mouth only. Occlude the opposite nostril with your finger while you form a tight seal with the mask around the mouth. Deliver short, abrupt bursts of ventilation through the mouth – be sure to maintain good seals with the mask and with your finger to the child’s nostril – until the foreign body is expulsed through the affected nostril.
This technique is an approximation of the above mother’s kiss technique – useful for unwilling parents or unsuccessful tries.10,25 The author prefers to position the child sitting up. A self-inflating bag-mask device is fitted with a very small mask: use an abnormally small mask (otherwise inappropriately small for usual resuscitative bag-mask ventilation) to fit over the mouth only. Choose an infant mask that will cover the child’s mouth only. Occlude the opposite nostril with your finger while you form a tight seal with the mask around the mouth. Deliver short, abrupt bursts of ventilation through the mouth – be sure to maintain good seals with the mask and with your finger to the child’s nostril – until the foreign body is expulsed through the affected nostril.
Beamsley Blaster (Continuous Positive Pressure) Technique
 For the very uncooperative child with a nasal foreign body amenable to positive pressure ventilation who fails the mother’s kiss and bag-mask technique, a continuous positive pressure method may be used. Connect one end of suction tubing to the male adaptor (“Christmas tree”) of an air or oxygen source. Connect the other end of the suction tubing to a male-to-male adaptor (commonly used for chest tube connections or connecting / extending suction tubes). Insert the end of the device into the child’s unaffected nostril. The air flow will deliver positive pressure ventilation continuously.
For the very uncooperative child with a nasal foreign body amenable to positive pressure ventilation who fails the mother’s kiss and bag-mask technique, a continuous positive pressure method may be used. Connect one end of suction tubing to the male adaptor (“Christmas tree”) of an air or oxygen source. Connect the other end of the suction tubing to a male-to-male adaptor (commonly used for chest tube connections or connecting / extending suction tubes). Insert the end of the device into the child’s unaffected nostril. The air flow will deliver positive pressure ventilation continuously.
With this technique there is a theoretical risk of barotrauma to the lungs or tympanic membranes. However, there is only one case reported in the literature of periorbital subcutaneous emphysema.
To minimize this risk, some authors recommend limiting to a maximum of four attempts using any positive pressure method.10
Nasal speculum
Optimize your visualization with a nasal speculum. The nostrils, luckily, will accommodate a fair amount of distention without damage.
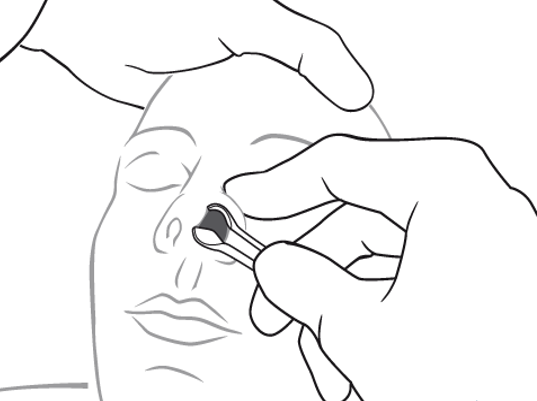 Hold the speculum vertically to avoid pressure on and damage to the vessel-and-nerve-rich nasal septum. Hold the handle of the speculum in the palm of your hand comfortably and while placing your index finger on the patient’s ala. This will help to control the speculum and your angle of sight. Your other hand is then free to use a hook or other tool for extraction.
Hold the speculum vertically to avoid pressure on and damage to the vessel-and-nerve-rich nasal septum. Hold the handle of the speculum in the palm of your hand comfortably and while placing your index finger on the patient’s ala. This will help to control the speculum and your angle of sight. Your other hand is then free to use a hook or other tool for extraction.
Lighting is especially important when using the nasal speculum: a focused procedure light or head lamp is very helpful. The author keeps a common camping LED headlamp in his bag for easy access.
Suction tips / catheters
Various commercial and non-commercial suction devices are on the market for removal of foreign bodies. All connect to wall suction, and vary by style, caliber of suction, and tip end interface. A commonly available suction catheter is the Frazier suction tip (right), a single-use device used in the operating room.
A modification to suction can be made with the Schuknecht foreign body remover (left; not to be confused with the suction catheter of the same name): a plastic cone-shaped tip placed on the end of the suction catheter to increase vacuum surface area and seal.
Laryngoscope and Magill Forceps
 If a child aspirates and occludes his airway, return to BLS maneuvers (as above). If the child becomes obtunded, use direct laryngoscopy to visualize the foreign body and remove with the Magill forceps. Hold the laryngoscope in your left hand as per usual. Hold the Magill forceps in your right hand – palm side down – to grasp and remove the foreign body.
If a child aspirates and occludes his airway, return to BLS maneuvers (as above). If the child becomes obtunded, use direct laryngoscopy to visualize the foreign body and remove with the Magill forceps. Hold the laryngoscope in your left hand as per usual. Hold the Magill forceps in your right hand – palm side down – to grasp and remove the foreign body.
Take-home Points
Beware the “vacuum palate”: a flat (especially clear plastic) foreign body hiding on the palate
Take seriously the complaint of foreign body without obvious evidence on initial inspection – believe that something is in there until proven otherwise
Control the environment, address analgesia and anxiolysis, have a back-up plan
Motto
Like a difficult airway: plan through the steps
MERCI – DANKE – Дякую – THANK YOU – GRACIAS – ありがとう— GRAZIE
Appendix A: Prevention
At the end of the visit, after some rapport has been established, counsel the caregivers about age-appropriate foods and “child-proofing” the home. This is a teachable moment – and only you can have this golden opportunity!
Age-appropriate foods
0-6 months: breastmilk or formula
6-9 months: introduce solid puree-consistency foods
9-12 months: small minced solids that require no chewing (well cooked, soft, chopped foods)
Although molars (required for chewing) erupt around 18 months, toddlers need to develop coordination, awareness to eat hard foods that require considerable chewing.
Not until 4 years of age (anything that requires chewing to swallow):
Hot dogs
Nuts and seeds
Chunks of meat or cheese
Whole grapes
Hard or sticky candy
Popcorn
Chunks of peanut butter
Chunks of raw vegetables
Chewing gum
Child-proofing the home
Refer parents to the helpful multi-lingual site from the American Academy of Pediatrics:
http://www.healthychildren.org/English/safety-prevention/at-home/Pages/Childproofing-Your-Home.aspx
An abbreviated list: use age-appropriate toys and “test” them out before giving them to your child to verify that there are no small, missing, or loose parts. Secure medications, lock up cabinets (especially with chemicals), do not store chemicals in food containers, secure the toilet bowl, and unplug appliances.
Parents should understand that “watching” their child alone cannot prevent foreign body aspiration: a recent review found that in 84.2% of cases, incidents resulting in an airway foreign body occurred in the presence of an adult.35
Best overall tip: get down on all fours and inspect your living area from the child’s perspective. It is amazing what you will find when you are least expecting it.
Appendix B: The Playbook's ENT Foreign Body Toolkit
Although your institution should supply you with what you need to deal with routine problems, we all struggle with having just what we need when we need it. High-volume disposable items such as cyanoacrylate (Dermabond), curettes, supplies for irrigation, alligator forceps, and the like certainly should be supplied by the institution. However, some things come in very handy as our back-up tools.
NB: we should be cognizant of the fact that tools that must be sterilized or autoclaved are not good candidates for our personal re-usable toolkits.
These items can all be found inexpensively – shop around online, or in home improvement stores:
- Head lamp, LED camping style: $5-15
- Neodymium magnet “pick-up tool”: $5-15
- Neodymium bar magnet: $6-20
- Wire, 24-gauge, spool of 25 yards (for snare technique): $6
- Day hook: $15-20
References
- Chapin MM, Rochette LM, Annest JL, Haileyesus T, Conner KA, Smith GA. Nonfatal Choking on Food Among Children 14 Years or Younger in the United States, 2001–2009. Pediatrics. 2013;132(2):275-281. doi:10.1542/peds.2013-0260.
- Committee on Injury V. Policy Statement—Prevention of Choking Among Children. Pediatrics. 2010:peds.2009-2862. doi:10.1542/peds.2009-2862.
- Brown L, Denmark TK, Wittlake WA, Vargas EJ, Watson T, Crabb JW. Procedural sedation use in the ED: management of pediatric ear and nose foreign bodies. Am J Emerg Med. 2004;22(4):310-314.
- Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76(8):1185-1189.
- DiMuzio J, Deschler DG. Emergency department management of foreign bodies of the external ear canal in children. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2002;23(4):473-475.
- Leffler S, Cheney P, Tandberg D. Chemical immobilization and killing of intra-aural roaches: an in vitro comparative study. Ann Emerg Med. 1993;22(12):1795-1798.
- Kiger JR, Brenkert TE, Losek JD. Nasal foreign body removal in children. Pediatr Emerg Care. 2008;24(11):785-792; quiz 790-792. doi:10.1097/PEC.0b013e31818c2cb9.
- Kadish HA, Corneli HM. Removal of nasal foreign bodies in the pediatric population. Am J Emerg Med. 1997;15(1):54-56.
- Tahir N, Ramsden WH, Stringer MD. Tracheobronchial anatomy and the distribution of inhaled foreign bodies in children. Eur J Pediatr. 2009;168(3):289-295. doi:10.1007/s00431-008-0751-9.
- Rempe B, Iskyan K, Aloi M. An Evidence-Based Review of Pediatric Retained Foreign Bodies. Pediatr Emerg Med Pract. 6(12).
- Digoy GP. Diagnosis and management of upper aerodigestive tract foreign bodies. Otolaryngol Clin North Am. 2008;41(3):485-496, vii - viii. doi:10.1016/j.otc.2008.01.013.
- Loren Yamamoto, Inaba A, DiMauro R. Radiologic Cases in Pediatric Emergency Medicine; University of Hawaii. Radiol Cases Emerg Med. http://www.hawaii.edu/medicine/pediatrics/pemxray/zindex.html. Accessed February 20, 2015.
- Painter K. Energizer makes button battery packages safer for kids. USA Today.
- ASGE Standards of Practice Committee, Ikenberry SO, Jue TL, et al. Management of ingested foreign bodies and food impactions. Gastrointest Endosc. 2011;73(6):1085-1091. doi:10.1016/j.gie.2010.11.010.
- Sharieff GQ, Brousseau TJ, Bradshaw JA, Shad JA. Acute esophageal coin ingestions: is immediate removal necessary? Pediatr Radiol. 2003;33(12):859-863. doi:10.1007/s00247-003-1032-4.
- Cohen S, Avital A, Godfrey S, Gross M, Kerem E, Springer C. Suspected Foreign Body Inhalation in Children: What Are the Indications for Bronchoscopy? J Pediatr. 2009;155(2):276-280. doi:10.1016/j.jpeds.2009.02.040.
- Haliloglu M, Ciftci AO, Oto A, et al. CT virtual bronchoscopy in the evaluation of children with suspected foreign body aspiration. Eur J Radiol. 2003;48(2):188-192. doi:10.1016/S0720-048X(02)00295-4.
- Jung SY, Pae SY, Chung SM, Kim HS. Three-dimensional CT with virtual bronchoscopy: a useful modality for bronchial foreign bodies in pediatric patients. Eur Arch Otorhinolaryngol. 2011;269(1):223-228. doi:10.1007/s00405-011-1567-1.
- Hussain SZ, Bousvaros A, Gilger M, et al. Management of ingested magnets in children. J Pediatr Gastroenterol Nutr. 2012;55(3):239-242. doi:10.1097/MPG.0b013e3182687be0.
- Brown JC, Otjen JP, Drugas GT. Too attractive: the growing problem of magnet ingestions in children. Pediatr Emerg Care. 2013;29(11):1170-1174. doi:10.1097/PEC.0b013e3182a9e7aa.
- Brown JC, Otjen JP, Drugas GT. Pediatric magnet ingestions: the dark side of the force. Am J Surg. 2014;207(5):754-759; discussion 759. doi:10.1016/j.amjsurg.2013.12.028.
- Menner AL. Pocket Guide to the Ear: A Concise Clinical Text on the Ear and Its Disorders. Thieme; 2011.
- Colina D, Dudek S, Lin M. Tricks of the Trade: ENT Dilemmas - How Do I Get That Out of There? ACEP News. http://www.acep.org/Clinical---Practice-Management/Tricks-of-the-Trade--ENT-Dilemmas---How-Do-I-Get-That-Out-of-There-/?__taxonomyid=118010. Published July 2009. Accessed February 5, 2015.
- Abadir WF, Nakhla V, Chong P. Removal of superglue from the external ear using acetone: case report and literature review. J Laryngol Otol. 1995;109(12):1219-1221.
- Kadish H. Ear and Nose Foreign Bodies “It is all about the tools.” Clin Pediatr (Phila). 2005;44(8):665-670. doi:10.1177/000992280504400803.
- Chisholm EJ, Barber-Craig H, Farrell R. Chewing gum removal from the ear using acetone. J Laryngol Otol. 2003;117(4):325. doi:10.1258/00222150360600995.
- White SJ, Broner S. The use of acetone to dissolve a Styrofoam impaction of the ear. Ann Emerg Med. 1994;23(3):580-582.
- Singer AJ, Sauris E, Viccellio AW. Ceruminolytic effects of docusate sodium: a randomized, controlled trial. Ann Emerg Med. 2000;36(3):228-232. doi:10.1067/mem.2000.109166.
- Bledsoe RD. Magnetically adherent nasal foreign bodies: a novel method of removal and case series. Am J Emerg Med. 2008;26(7):839.e1-e839.e2. doi:10.1016/j.ajem.2008.01.036.
- Dolderer JH, Kelly JL, Morrison WA, Penington AJ. FOREIGN-BODY RETRIEVAL USING A RARE EARTH MAGNET: Plast Reconstr Surg. 2004;113(6):1869-1870. doi:10.1097/01.PRS.0000119869.01081.1C.
- Yeh B, Roberson JR. Nasal magnetic foreign body: a sticky topic. J Emerg Med. 2012;43(2):319-321. doi:10.1016/j.jemermed.2010.02.013.
- Fundakowski CE, Moon S, Torres L. The snare technique: a novel atraumatic method for the removal of difficult nasal foreign bodies. J Emerg Med. 2013;44(1):104-106. doi:10.1016/j.jemermed.2012.07.070.
- Chan TC, Ufberg J, Harrigan RA, Vilke GM. Nasal foreign body removal. J Emerg Med. 2004;26(4):441-445. doi:10.1016/j.jemermed.2003.12.024.
- Cook S, Burton M, Glasziou P. Efficacy and safety of the “mother’s kiss” technique: a systematic review of case reports and case series. Can Med Assoc J. 2012;184(17):E904-E912. doi:10.1503/cmaj.111864.
- Gregori D, Morra B, Snidero S, et al. Foreign bodies in the upper airways: the experience of two Italian hospitals. J Prev Med Hyg. 2007;48(1):24-26.
This post and podcast are dedicated to Linda Dykes, MBBS(Hons) for her can-do attitude and collaborative spirit. Thank you for sharing your knowledge, experience, and heart with the world.
When you give only after you're asked, you've waited too long.
– John Mason
First, learn to bag
Place a towel roll under the scapulae to align oral, pharyngeal, and tracheal axes:
Use airway adjuncts such as the oropharyngeal airway or a nasal trumpet.
Use the two-hand ventilation technique whenever possible:
(See Adventures in RSI for more)
Supraglottic Airways:
for difficult bag-valve-mask ventilation or a difficult airway
(details in audio)
LMA Classic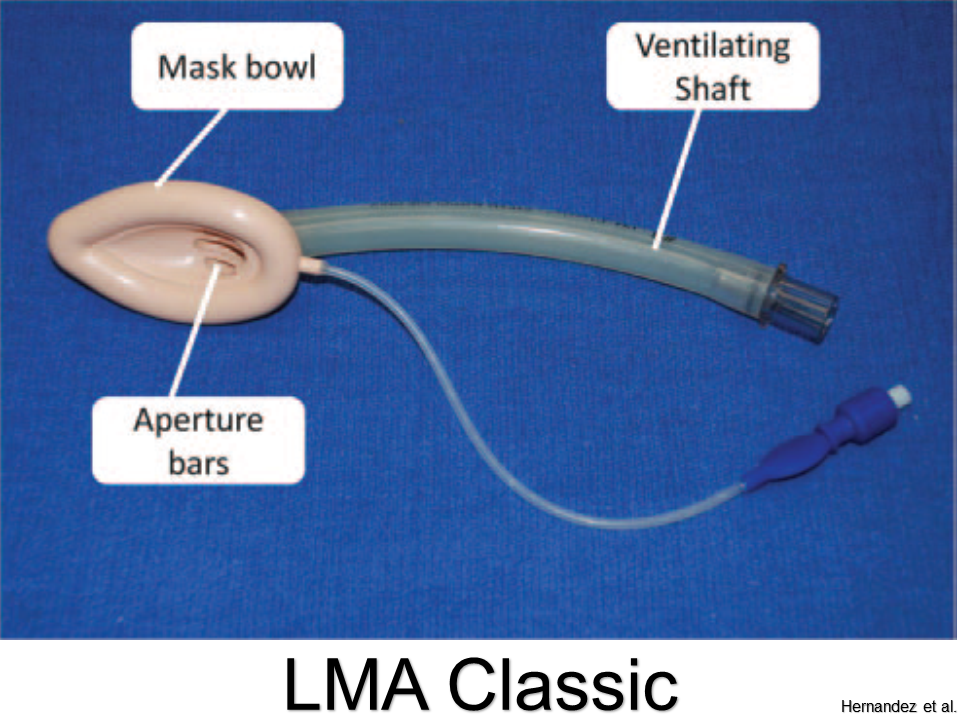
Pros: Best studied; sizes for all ages
Cons: Cannot intubate through aperture
LMA Supreme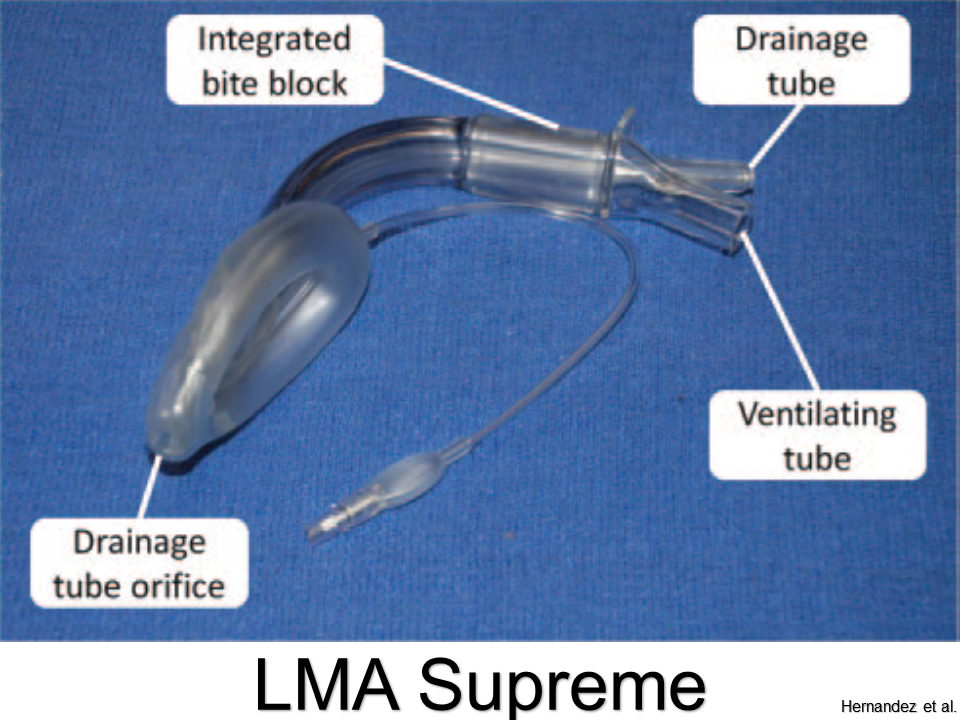
Pros: Better ergonomics with updated design; bite bloc; port for decompression
Cons: Cannot pass appropriate-sized ETT through tube
King Laryngeal Tube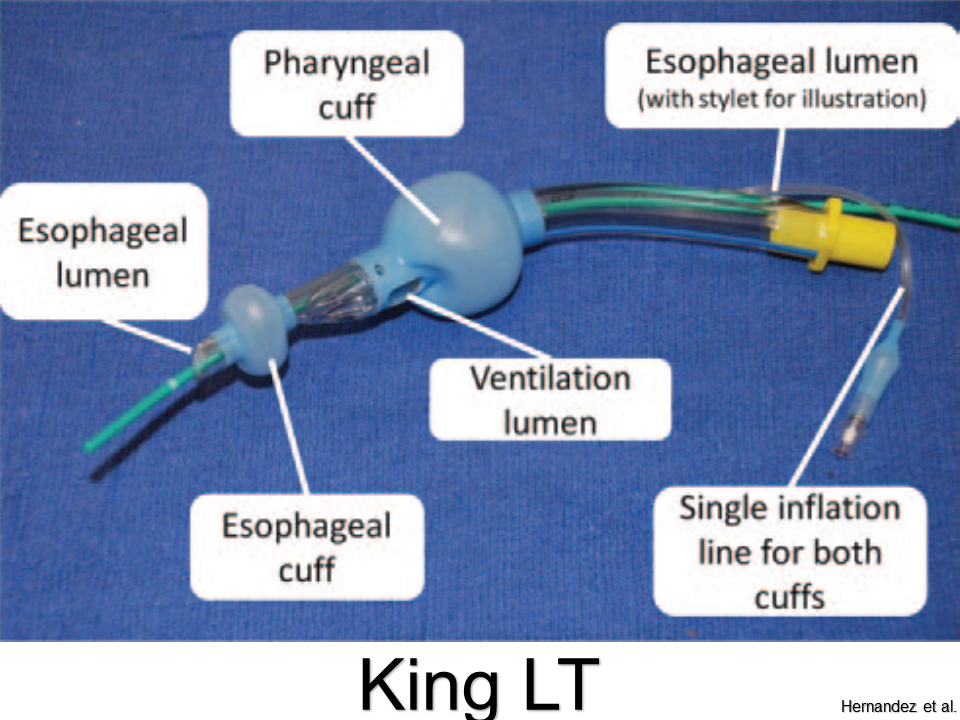
Pros: Little training needed; high success rate; single inflation port
Cons: Flexion of tube can impede ventilation or cause leaks; only sized down to 12 kg (not for infants and most toddlers)
Air-Q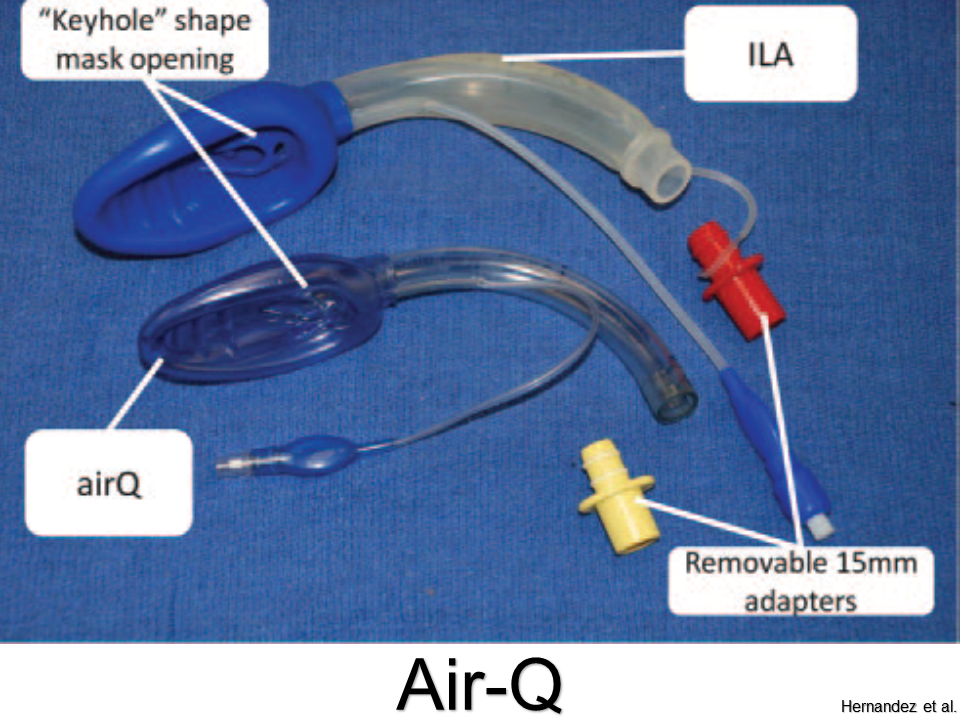
Pros: Easy to place; can intubate through aperture
Cons: Not for neonates less than 4 kg
iGel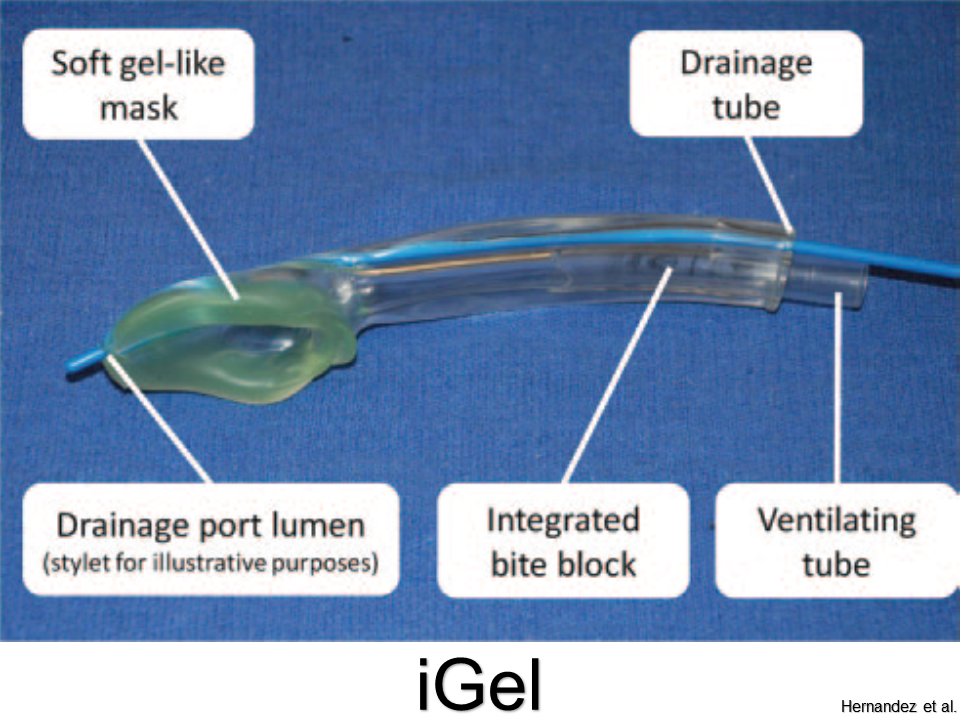
Pros: Molds more accurately to supraglottis; no need to inflate; good seal pressures
Cons: Cannot intubate through (without fiberoscopy)
Summary
• If you can bag the patient, you're winning.
• If you have difficulty bagging, or anticipate or encounter a difficult airway, then don't forget your friend the supraglottic airway (SGA).
• Ego is the enemy of safety: SGAs are simple, fast, and reliable.
• Just do it.
References
Supraglottic Airway on WikEM
This post and podcast are dedicated to Tim Leeuwenburg, MBBS FRACGP FACRRM DRANZCOG DipANAES and Rich Levitan, MD, FACEP for keeping our minds and our patients' airways -- open. You make us better doctors. Thank you.
Powered by #FOAMed — Tim Horeczko, MD, MSCR, FACEP, FAAP
Pediatric; Emergency Medicine; Pediatric Emergency Medicine; Podcast; Pediatric Podcast; Emergency Medicine Podcast; Horeczko; Harbor-UCLA; Presentation Skills; #FOAMed #FOAMped #MedEd
When should you commit to getting urine?
When can you wait?
When should you forgo testing altogether?
When do I get urine?
Symptoms – either typical dysuria, urgency, frequency in a verbal child, or non-descript abdominal pain or vomiting in a well appearing child.
Fever – but first look for an obvious alternative source, especially viral signs or symptoms.
No obvious source?
Risk stratify before “just getting a urine”.
In a low risk child, with obviously very vigilant parents, who is well appearing, you may choose not to test now, and ensure close follow up.
Bag or cath?
The short answer is: always cath, never bag.
(Pros and cons in audio)
What is the definition of a UTI?
According to the current clinical practice guideline by the AAP, the standard definition of a urinary tract infection is the presence of BOTH pyuria AND at least 50 000 colonies per mL of a single uropathogen.
Making the diagnosis in the ED:
The presence of WBCs with a threshold of 5 or greater WBCs per HPF is required.
What else goes into the urinalysis that may be helpful?
Pearl: nitrites are poorly sensitive in children. It takes 4 hours for nitrites to form, and most children this age do no hold their urine.
Pearl: the enhanced urinalysis is the addition of a gram stain. A positive gram stain has a LR+ of 87 in infants less than 60 days, according to a study by Dayan et al. in Pediatric Emergency Care.
When can I just call it pyelonephritis?
In an adult, we look for UTI plus evidence of focal upper tract involvement, like CVA tenderness to percussion or systemic signs like nausea, vomiting, or fever. It is usually straightforward.
It’s for this reason that the literature uses the term “febrile UTI” for children. Fever is very sensitive, but not specific in children.
The ill-appearing child has pyelonephritis. The well-appearing child likely has a “febrile UTI”, without upper involvement. However, undetected upper tract involvement may be made in retrospect via imaging, if done.
How should I treat UTIs?
For simple lower tract disease, treat for at least 7 days. There is no evidence to support 7 versus 10 versus 14 days. My advice: use 7-10 days as your range for simple febrile UTI in children.
Pyelonephritis should be treated for a longer duration. Treat pyelonephritis for 10-14 days.
What should we give them?
Sulfamethoxazole and trimethoprim (Bactrim) is falling out of favor, mostly because isolates in many communities are resistant. There is an association of Stevens-Johnson Syndrome (SJS) with Bactrim use. This may be confounded by its prior popularity; any antibiotic can cause SJS, but there are more case reports with Bactrim.
Cephalexin (Keflex): 25 mg/kg dose, either BID or TID. It is easy on the stomach, rarely interacts with other meds, has high efficacy against E. coli, and most importantly, cephalexin has good parenchymal penetration.
Nitrofurantoin is often used in pregnant women, because the drug tends to concentrate locally in the urine. However, blood and tissue concentrations are weak. It may be ineffective if there is some sub-clinical upper tract involvement.
Cefdinir is a 3rd generation cephalosporin available by mouth, given at 14 mg/kg in either one dose daily or divided BID, up to max of 600 mg. This may be an option for an older child who has pyelonephritis, but is well enough to go home.
Whom should we admit?
The first thing to consider is age. Any infant younger than 2 months should be admitted for a febrile UTI. Their immune systems and physiologic reserve are just not sufficient to localize and fight off infections reliably.
The truth is, for serious bacterial illness like pneumonia, UTI, or severe soft tissue infections, be careful with any infant less than 4-6 months of age.
Of course, the unwell child – whatever his age – he should be admitted. Think about poor feeding, irritability, dehydration – in that case, just go with your gut and call it pyelonephritis, and admit.
What is the age cut-off for a urine culture?
In adults, we think of urine culture only for high-risk populations, such as pregnant women, the immunocompromised, those with renal abnormalities, the neurologically impaired, or the critically ill, to name a few.
In children, it’s a little simpler. Do it for everyone.
Who is everyone? Think of the urine rule of 10s:
10% of young febrile children will have a UTI
10% of UAs will show no evidence of pyuria
Routine urine culture in all children with suspected or confirmed UTI up to about age 10
What do I do then with urine culture results?
From a quality improvement and safety perspective, consider making this a regular assignment to a qualified clinician.
Check once in 24-48 hours to find possible growth of a single uropathogen with at least 50 000 CFU/mL. Look at the record to see that the child is one some antibiotic, or the reason why he may not. Call the family if needed.
A second check at 48-72 hours may be needed to verify speciation and sensitivities.
The culture check, although tedious, is important to catch those small children who did not present with pyuria and who may need antibiotics, or to verify that the right agent is given.
Ok, so your UA is negative…now what?
The culture is cooking, but you are not convinced. Below is the differential diagnosis for common causes of pyuria in children: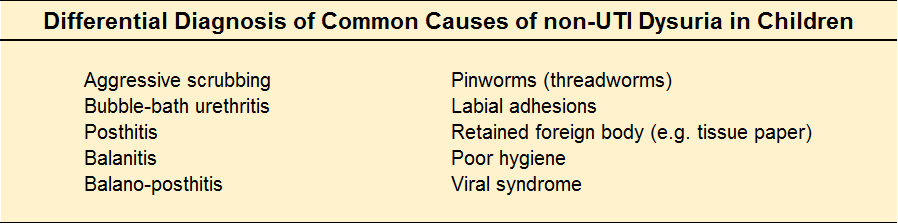
What kind of follow-up should the child get?
The younger the child, the more we worry about missing a decompensation. Encourage the parents to call the child's primary care clinician for a re-check in a few days, and to discuss whether or not further work-up such as imaging is indicated. As always, strict return to ED precautions are helpful.
Who needs imaging?
A more accurate question is: what is an important anomaly to detect?
Vesiculo-ureteral reflux – a loose ureteropelvic junction causes upstream reflux when the bladder constricts.
Uretero-pelvic junction obstruction – in older children or young adults with hematuria, UTI, abdominal mass, or pain. Infants born with UPJ obstruction have congenital hydronephrosis.
Ureterocoele – a cystic mass in the bladder. It is not malignant, but can cause ureteral dilation, and hydronephrosis. Treatment is surgical.
Ectopic ureter – either a duplication of the draining system, or an abnormal connection, such as the epidydimis or cervix.
Posterior urethral valves – occur only in boys, and they are a bit of a misnomer. The most common type of congenital bladder outlet obstruction, posterior urethral valves are just extra folds of membrane in the lumen of the prostatic urethra. Usually ablation by cystoscopy does the trick.
Urachal remnant – a leftover from fetal development, and an abnormal connection between the bladder and the umbilicus. Look for an “always wet” belly button in an infant, or an umbilical mass with pain and fever in an older child.
Imaging of choice as an outpatient?
Renal and bladder ultrasound (RBUS) after the first UTI is recommended (although incompletely followed in practice).
If the RBUS is positive, or with the second UTI, DMSA scan to evaluate possible renal scarring.
So, with all of this testing – are we over doing it?
Like anything, it’s a balance. A few tips to avoid iatrogenia by way of a summary.
If a child over 3 months of age is well, has no comorbidities, has a low grade fever "in the 38s" (38-38.9 °C) without a source, especially if less than 24 hours, you are very safe to do watchful waiting at home.
More to the point, an otherwise well child with an obvious upper respiratory tract infection has a source of his fever.
If your little patient has risk factors for UTI, or you are otherwise concerned, send the UA and send the culture. You can opt out of the culture by middle school in the otherwise healthy child.
And finally, deputize parents to carry the ball from here – the child needs ongoing primary care and his pediatrician may elect to do some screening. Don’t promise or prime them for it – rather, encourage the conversation.
BONUS:
Suprapubic aspiration (details in podcast audio; video below)
BONUS BONUS:
Infant Clean Catch Technique
Step One: feed the baby, wait twenty minutes.
Step Two: clean the genitals with soap and warm water and dry with gauze. Have your sterile urine container open and at the ready.
Step Three: one person holds the baby under his armpits with his legs dangling. The other person gently taps the bladder (100 taps/min), then massages the lower back for 30 seconds.
Step Four: Clean Catch! (can also repeat process)
References
Downs SM. UTI and watchful waiting: the courage to do nothing. Pediatrics. 2014 Mar;133(3):535-6.
This post and podcast are dedicated to Brad Sobolewski, MD, MEd for his innovation and tenacity in all things #FOAMed, #FOAMped, and #MedEd. Thanks, Brad, for your enthusiasm, energy, and for your fantastic PEM Currents and PEM Blog.
Pediatric Urinary Tract Infections
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
N.B.: This month's show notes are a departure from the usual summary. Below is a reprint (with permission) of a soon-to-be released chapter, Horeczko T. "Acute Pain in Children". In Management of Pain and Procedural Sedation in Acute Care. Strayer R, Motov S, Nelson L (eds). 2017. Rather than the customary blog post summary, the full chapter (with links) is provided as a virtual reference.
INTRODUCTION
Pain is multifactorial: it is comprised of physical, psychological, emotional, cultural, and contextual features. In children often the predominant feature may not be initially apparent. Although clinicians may focus on the physical component of pain, much time, energy, and suffering can be saved through a holistic approach. What is the age and developmental stage of the child? How is the child reacting to his condition? What are the circumstances? What is the family or caregiver dynamic?
We rely much on how patients and families interact with us to gauge pain. Assessing and managing children’s pain can be challenging, because they may not exhibit typically recognized signs and symptoms (Srouji 2010). Further, children participate in and absorb their family’s culture and specific personality from a very young age (Finley 2009). Knowing the context of the episode may help. For example, a very anxious caregiver can easily transmit his or her anxiety to the child, which may either inhibit or amplify presentation of symptoms (Bearden 2012).
The guiding principles in pediatric pain assessment and management are: know the child; know the family; and know the physiology. Children have long suffered from an under-treatment of their pain, due both to our incomplete acknowledgement of their pain and our fear of treatment (Howard 2003). As the pendulum on pain management swings one way or the other, do not let your pediatric patient get knocked by the wayside. Take a thoughtful approach: know the signs and symptoms, and aggressively treat and reassess.
ASSESSMENT
Each stage of development offers a unique framework to the child’s signs and symptoms of pain. In pre-verbal children, use your observational skills in addition to the parent’s report of behavior. Verbal children can self-report; younger children require pictorial descriptions, while older children and adolescents may use standard adult scales. In all ages, ask open-ended questions and allow the child to report and speak for himself whenever possible.
Neonates
Neonates are a unique group in pain assessment. The neonate (birth to one month of age) has not yet acquired social expression of pain, and his nascent nervous system is only now learning to process it. Do not expect typical pain behaviors in neonates. Facial grimacing is a weak indicator of pain in neonates (Liebelt 2000). When this behavior is present, look for a furrowed brow, eyes squeezed shut, and a vertically open mouth. Tachycardia, tachypnea, and a change in behavior can be indicators not only to the presence of pain, but possibly to its etiology as well.
Neonatal observational scales have been validated in the intensive care and post-operative settings; ED-specific quantitative scales are lacking. CRIES is a 10-point scale, using a physiologic basis similar to APGAR: Crying; Requires increased oxygen administration (distress and breath-holding); Increased vital signs; Expression; and Sleeplessness (Krechel 1995). CRIES (Table 1) was validated for post-operative patients; to adapt its use for the ED, the most conservative approach is to substitute “preoperative baseline” with normal range for age. Although the numerical values of CRIES have not been validated to date in the ED, the clinician may find the domains included in CRIES to be a useful cognitive construct in assessing neonatal pain.
Neonatal pain pathways are particularly plastic; prompt assessment of and increased alertness to neonatal pain may help to mitigate long-lived pain sensitivity and hyperalgesia (Taddio 2002). In other words, treat the neonate’s pain seriously, as you may save him long-term pain sequelae in the future.
Infants and Toddlers
This group will begin to exhibit more reproducible, reliable signs and symptoms of pain.
For infants of less than one year of age, the Neonatal Infant Pain Scale (NIPS) uses observational and physiologic parameters to detect pain (Table 2). A score of 0-2 indicates no pain present. A score of 3-4 indicates mild to moderate pain; non-pharmacologic techniques may be tried first. A score of 5 or greater indicates severe pain; some pharmacologic intervention is indicated (Lawrence 1993).
For children greater than one year who are preverbal, a well performing scale is the FLACC score: Face, Legs, Activity, Cry, Consolability (Table 3).
Contextual and caregiver features predominate in this group. Frequent reassessments are helpful, as the initial trepidation and fright in triage may not accurately reflect the child’s overall pain status.
Preschool and School-age children
Increasing language development offers the hope of more information to the clinician, but be careful not to ask leading questions. Do not jump directly to “does this hurt?”. Preschoolers will say ‘yes’ to anything, in an attempt to please you. School-age children may passively affirm your “statement”, if only to validate their human need for care or attention. Start with some ice-breaking banter, lay down the foundations for rapport, and then ask open-ended questions. Be careful not to allow the caregiver to “instruct” the child to tell you where it hurts, how much, how often, etc. Rather, engage the parents by asking them what behavior they have noticed. Eliciting history from both the child and the parent will go a long way in constructing a richer picture of the etiology and severity of the pain, and will help to build rapport and trust.
The Baker-Wong FACES Pain Rating scale (Figure 1) was developed with feedback from children and has been validated for use in those 3 years of age and older (Keck 1996, Tomlinson 2010).
Adolescents
Adolescents vary in their development, maturity, and coping mechanisms. You may see a mixture of childhood and adult behaviors in the same patient; e.g. he may be initially stoic or evades questioning, then later exhibits pseudo-inconsolability. Do what you can to see the visit from the adolescent’s perspective, and actively transmit your concern and intention to help – many will respond to a warm, open, non-judgemental, and helpful attitude. The overly “tough” adolescent is likely secretly fearful, and the “dramatic” adolescent may simply be very anxious. Take a moment to gauge the background behind the presentation.
You may use the typical adult scale of 0 (no pain) to 10 (worst pain), or the Faces Pain Scale–Revised (FPS-R). The FPS-R uses more neutral and realistic faces and, unlike the Wong Baker scale, does not use smiling or crying faces to anchor the extremes of pain (Tsze 2013).
PAIN PHYSIOLOGY
Pain includes two major components: generation and perception. Generation of pain involves the actual propagation of painful stimuli, either through nociceptive pain or neuropathic pain. Nociceptive pain arises from free nerve endings responding to tissue damage or inflammation.
Nociceptive pain follows a specific sequence: transduction (an action potential triggered by chemical mediators in the tissue, such as prostaglandins, histamine, bradykinin, and substance P); transmission (the movement of the action potential signal along the nerve fibers to the spinal cord); perception (the impulse travels up the spinothalamic tract to the thalamus and midbrain, where input is splayed out to the limbic system, somatosensory cortex, and parietal and frontal lobes); and modulation (the midbrain enlists endorphins, enkephalins, dynorphin, and serotonin to mitigate pain) (Pasero 2011). As clinicians we can target specific “stations” along the pain route to target the signal more effectively.
Simple actions such as ice, elevation, local anesthetics, or splinting help in pain transduction. Various standard oral, intranasal, or IV analgesics may help with pain’s transmission. Non-pharmacologic techniques such as distraction, re-framing, and others can help with pain perception. The sum of these efforts encourage pain modulation.
A phenomenon separate from nociceptive pain is neuropathic pain, the abnormal processing of pain stimuli. It is a dysregulated, chaotic process that is difficult to manage in any setting. Separating nociceptive from neuropathic symptoms may help to select specific pain treatments and to clarify treatment goals and expectations.
Neonates
Neonates are exquisitely sensitive to many analgesics. Hepatic enzymes are immature and exhibit decreased clearance and prolonged circulating levels of the drug administered. Once the pain is controlled, less frequent administration of medications, with frequent reassessments, are indicated.
The neonate’s vital organs (brain, heart, viscera) make up a larger proportion of his body mass than do muscle and fat. That is to say, the volume of distribution is unique in a neonate. Water-soluble drugs (e.g. morphine) reach these highly perfused vital organs quickly; relatively small overdosing will have rapid and exaggerated central nervous system and cardiac effects. The neonate’s small fat stores and muscle mass limit the volume of distribution of lipophilic medications (e.g. fentanyl, meperidine), also making them more available to the central nervous system, and therefore more potent. Other factors that predispose neonates to accidental analgesic overdose are their decreased concentrations of albumin and other plasma proteins, causing a higher proportion of unbound drug. Renal clearance is also decreased in the first few months of life.
Clinical note: in the ED, neonates often require analgesia for procedures more than for injury. Non-pharmacologic techniques predominate (see below). Make liberal use of local anesthetics such as eutectic mixture of local anesthetics (EMLA; for intact skin, e.g. IV access, lumbar puncture) and lidocaine-epinephrine-tetracaine gel (LET; for superficial open skin and soft tissue application). Oral sucrose (30%) solutions (administered either with a small-volume syringe or pacifier frequently dipped in solution) are effective for minor procedures (Harrison 2010, Stevens 2013) via the release of dopamine and through distraction by mechanical means. Neonates with severe pain may be managed with parenteral analgesics, on a monitor, and with caution.
Infants and Toddlers
With increasing body mass comprised of fat stores in conjunction with an increase in metabolism, this group will require a different approach than the neonate. For many medications, these children will have a greater weight-normalized clearance than adults (Berde 2002). They will often require more frequent dosing. Infants and toddlers have a larger functioning liver mass per kilogram of body weight, with implications for medications cleared by cytochrome p-450.
Clinical note: some drugs, such as benzodiazepines, will have both a per-kilogram dosing as well as an age-specific modification. When giving analgesics or anxiolytics to young children, always consult a reference for proper dosing and frequency.
School-age children and Adolescents
This group retains some hyper-metabolic features of younger children, but the dose-effect relationship is more linear and transparent. Physiologic clearance is improved, and from a physical standpoint, these are typically lower-risk children. From a psychological standpoint, this group may need more non-pharmacologic consideration and support to modulate pain optimally.
NON-PHARMACOLOGIC TREATMENT
The first line of treatment in all pain management is non-pharmacopeia (Horeczko 2016). Not only is this the safest of all techniques, but often the most effective. Some are simple comfort measures such as splinting (fracture or sprain), applying cold (acute soft tissue injury) or heat (non-traumatic, non-specific pain), or other targeted non-pharmacology.
Many a pain control regimen is sabotaged without consideration of non-pharmacologic techniques, which may augment, or at times replace, analgesics. Think of non-pharmacopoeia as your “base coat” or “primer” before applying additional coats of analgesic treatment. With the right base coat foundation, you have a better chance of painting a patient’s symptoms a more tolerable and long-lasting new color.
A tailored approach based on age will allow the practitioner to employ a child’s developmental strengths and avoid the frustration that results in asking the child to do what he is not capable of doing. A brief review of Piaget’s stages of development will help to meet the child at his developmental stage for best effect (Piaget 1928, Sheppard 1977) during acute painful presentations and minor procedures.
Sensorimotor stage (from birth to age 2): Children use the five senses and movement to explore the world. They are egocentric: they cannot see the world from another’s viewpoint. At 6 to 9 months, object permanence is established: understanding that objects (or people) exist even without seeing them.
Preoperational stage (from ages 2 to 7): Children learn to use language. Magical thinking predominates. They do not understand rational or logical thinking.
Concrete operational stage (from age 7 to early adolescence): Children can use logic, but in a very straightforward, concrete manner (they do well with simple examples). By this stage, they move from egocentrism to understanding another point of view. N.B. Some children (and adults) never completely clear this stage.
Formal operational stage (early adolescence to adult): children are capable of abstract thinking, rationalizing, and logical thinking.
It is important to assess the child’s general level of development when preparing and guiding him through the minor procedure or distracting him until his pain is controlled. It is not uncommon for acutely ill or injured to regress temporarily in their behavior (not their development) as a coping mechanism.
Neonate and Infant (0-12 months)
Involve the parent, and have the parent visible to the child at all times if possible. Make advances slowly, in a non-threatening manner; limit the number of staff in the room. Use soothing sensory measures: speak softly, offer a pacifier, and stroke the skin softly. Swaddle the infant and encourage the parent to comfort him during and after the procedure. Engage their developing sensorimotor skills to distract them.
Toddler to Preschooler (1-5 years)
Use the same techniques as for the infant, and add descriptions of what he will see, hear, and feel; you can use a doll or toy to demonstrate the procedure. Use simple, direct language, and give calm, firm directions, one at a time. Explain what you are doing just before doing it (do not allow too much time for fear or anxiety to take root). Offer choices when appropriate; ignore temper tantrums. Distraction techniques include storytelling, bright and flashy toys, blowing bubbles, pinwheels, or having another staff member play peek-a-boo across the room. The ubiquitous smart phone with videos or games can be mesmerizing at this age.
School age (6-12 years)
Explain procedures using simple language and (briefly) the reason (understanding of bodily functions is vague in this age group). Allow the child to ask questions, and involve him when possible or appropriate. Distraction techniques may include electronic games, videos, guided imagery, and participation in the minor procedure as appropriate.
Adolescent (13 and up)
Use the same techniques for the school age child, but can add detail. Encourage questioning. Impose as few restrictions as possible – be flexible. Expect more regression to childish coping mechanisms in this age group. Distraction techniques include electronic games, video, guided imagery, muscle relaxation-meditation, and music (especially the adolescent’s own music, if available).
APPLIED PHARMACOLOGY
No amount of knowledge of the above physiology, pharmacology, or developmental theory will help your little patient in pain without a well constructed and enacted plan. Aggressively search out and treat your pediatric patient’s presence and source of pain. Frequent reassessments are important to ensure that breakthrough pain treatment is achieved, when re-administration is indicated, or when a change of plan is necessary. This is the time to involve the parents or caregivers to let them know what the next steps are, and what to expect.
Start with the least invasive modality and progress as needed. After non-pharmacologic treatments such as splinting, ice, elevation, distraction, and guided imagery, have an escalation of care in mind (Figure 2).
From a pharmacologic perspective, various options are available. Your pain management plan will differ depending on whether a painful procedure is performed in the ED (Table 4). Once pain is addressed, create a plan to keep it managed. Consider the trajectory of illness and the expected time frame of the painful episode. Include practicalities such as how well the pain may be controlled as an outpatient. Poorly controlled pediatric pain is more often managed as an inpatient than the same condition in an adult. Speak frankly with the parents about what drug is indicated for what type of pain and that treatment goals typically do not include absence of all pain, but function in face of the pain, in anticipation for clinical improvement.
A special note on codeine: Tylenol with codeine (“T3”) has never been a very effective pain medication, as up to 10% of patients lack enzymatic activity to metabolize it into morphine, its active form (Crews 2014). New evidence is emerging on the erratic and unpredictable individual metabolism of codeine. Some children are ultra-rapid-metabolizers of codeine to morphine, causing a rapid “bolus” of the available drug, with respiratory depression and death in some cases (Ciszkowski 2009, Racoosin 2013). Author’s advice: take codeine off your formulary.
COMMON SCENARIOS
Head and neck pain
Most common non-traumatic head and neck complaints can be managed non-pharmacologically (e.g. headache: improved hydration, sleep, stress, nutrition) or with PO medications, such as NSAIDs. The anti-inflammatory nature of ibuprofen (10 mg/kg PO q 4-6 h prn, up to adult dose) for example, will treat the cause as well as the symptoms of ear pain, sore throat, and muscular pain. Ibuprofen may be more effective than acetaminophen (paracetamol) for odontogenic pain (Bailey 2013). For most applications, acetaminophen may be as effective; however, the combination of both NSAIDs is not likely to be more effective than either agent individually (Merry 2013).
True migraine headache may be treated with all of the above, and rescue therapy may include prochlorperamide (0.15 mg/kg IV, up to 10 mg ) (Brousseau 2004), often given with diphenhydramine (1 mg/kg PO or IV, up to 50 mg) and IV fluids. Ketoralac (0.5 mg/kg IV, up to 10 mg) may be substituted for ibuprofen (Paniyot 2016). Other specific therapies may be considered, although evidence for them varies.
Chest pain
After ruling out important pulmonary (e.g. the under-recognized spontaneous pneumothorax) and cardiac (e.g. pericarditis, myocarditis) etiologies, many chest complaints are amenable to NSAIDs. There is often a large component of anxiety in the child and/or parents in chest pain; no amount of medication will assuage them without addressing their concerns as well.
Abdominal pain
Abdominal pain in children is challenging, as it is common, often benign, but may be disastrous if the etiology is missed. For mild pain, consider acetaminophen as indicated (15 mg/kg/dose q 4-6 h prn, up to 650 mg). The oral route is preferred, but intravenous acetaminophen is an option for patients unable to tolerate PO, or for those in whom the per rectum (PR) route is contraindicated (e.g. neutropenia) (Babl 2011, Dokko 2014). For children with moderate to severe abdominal pain in whom a nil per os (NPO) status is ideal, consider rehydration/volume repletion, and small, frequent aliquots of a narcotic agent. Surgical pain is not “erased” by opioids (Thomas 2003, Poonai 2014); treating pain improves specificity to certain surgical emergencies with retained diagnostic accuracy (Manterola 2007). If there is inter-departmental concern about prolonged effects, sedation, limitation in the physical exam, or there is a need to “see if the pain will come back”, you may opt to use fentanyl initially for its shorter half-life. More frequent re-assessments may help the surgical team in its deliberations. Transition quickly to a longer-acting opioid as soon as possible.
Long-bone injuries
Fracture pain should be addressed immediately with splinting and analgesia. Oral, intranasal, and intravenous routes are all acceptable, depending on the severity of the injury and symptoms.
Intranasal (IN) medications offer the advantage of a fast onset for moderate-to-severe pain (Graudins 2015), either as monotherapy or as a bridge to parenteral treatment (Table 4). The ideal volume of IN medication is 0.25 mL/naris, with a maximum of 1 mL/naris. Common concentrations of fentanyl limit its mg/kg use to the school-aged child; intranasal ketamine may be used for pain (i.e. sub-dissociative dose) up to adult weight.
Long-bone injuries are a good opportunity to employ a speedy modality that requires little technical skill in administration: nebulized fentanyl. Clinically significant improvement in pain scales are achieved with 3 mcg/kg/dose of fentanyl administered via standard nebulizer in children 3 years of age or older (Miner 2007, Furyk 2009). Nebulized fentanyl is a rapid, non-invasive alternative to the IN route for older children, adolescents, or adults, in whom the volume of IN medication would exceed the recommended per naris volume (Deaton 2015).
Consider an aggressive, multi-modal approach to control symptom up front. For example, for a simple forearm fracture, you may opt to give an oral opioid, perform a hematoma block, and offer inhaled nitrous oxide for reduction, rather than a formal intravenous procedural sedation (Luhmann 2006).
Ultrasound-guided peripheral nerve blocks are a good pain control adjunct, after initial treatment, and in communication with referring consultants (Ganesh 2009, Suresh 2014).
Skin and Soft tissue
Skin and soft tissue injuries or abscesses often require solid non-pharmacopoeia in addition to local anesthetics. For IV cannulation, consider EMLA if the patient is stable and a minor delay is acceptable.
Topical ethyl chloride vapo-coolant offers transient pain relief due to rapid cooling and may be used just prior to an IV start (Farion 2008). Try this: engage your young child’s imagination to distract him and say, “have you ever held a snow ball? You are in luck – it’s just like that – here, do you feel it?”.
Vibratory adjuncts such as the “BUZZY” bee can be placed near the IV cannulation site to provide mechanical and cognitive distraction (Moadad 2016).
Needleless lidocaine injectors may facilitate IV placement without obscuring the target vein (Spanos 2008, Lunoe 2015). The medication is propelled into the dermis by a CO2 cartridge that makes a loud popping sound; try this to alleviate anxiety, just before using it: “your skin looks thirsty – it needs a drink – there you are!”.
As with any minor procedure, when you tell the child what you are doing, be sure to do it right away. Do not delay or build suspense.
Lidocaine-epinephrine-tetracaine gel (LET) is used for open or mucosal wounds. Apply as soon as possible in the visit. The goal of LET is to pretreat the wound to allow for a painless administration of injectable anesthetic. A common practice to apply LET two or three times at 15-minute intervals for deeper anesthesia, in an attempt to avoid injection altogether. Researchers are currently working to offer an evidence base to this anecdotal practice.
Pediatric burns should be assessed carefully and treated aggressively. Submersion of the affected extremity in room-temperature water (if possible) or applying room-temperature saline-soaked gauze will both thwart ongoing thermal damage, soothe the wound, and provide foundational first-aid. Minor burns can be treated topically and with oral medications. Major burns require IN, IM, or IV analgesics with morphine. Treatment may escalate to ketamine (Gandhi 2010), in analgesic or dissociative dosing, depending on the context. Post-traumatic disorders are common in burns; effective pain management is ever-more important in these cases.
SPECIFIC SCENARIOS
The child with chronic medical problems
Children with acute exacerbations of their chronic pain or episodic painful crises require special attention. Some examples of children with recurring pain are those suffering from sickle cell disease, juvenile idiopathic arthritis, complex regional pain syndrome, and cancer. Find out whether these symptoms and circumstances are typical for them, and what regimen has helped in the past. Previous unpleasant experiences may prime these children with amplified anxiety and perception of pain (Cornelissen 2014). Target the disease process and do your best to show the patient and his family you understand his condition and needs.
An equally challenging scenario is the child with chronic pain. Treat the entire patient with a multimodal approach. Limit opioids as possible. As an opioid-sparing strategy or as rescue therapy, consider sub-dissociative ketamine, especially for conditions such as sickle cell crisis, complex regional pain syndrome, autoimmune disorders, or chronic pain due to sub-acute trauma (Sheehy 2015). Intranasal ketamine may be used for sub-dissociative pain control at 0.5 – 1 mg/kg (Andolfatto 2013, Yeaman 2013). Intravenous infusions of ketamine at 0.1 – 0.3 mg/kg/h may be initiated in the ED and continued 4 – 8 h/d, up to a maximum of 16 h total in 3 consecutive days (Sheehy 2015). In vaso-occlusive episodes, dexmedetomidine has been shown to be an effective adjunct for severe pain poorly responsive to opioids and/or ketamine (Sheehy 2015b).
The child with cognitive impairment
Children with cognitive impairment such as those with various genetic or metabolic syndromes, or primary neurologic conditions such as some with cerebral palsy are a challenge to assess and treat properly. These children not only cannot explain their symptoms, but they also have atypical expressions of pain. Pain responses in severely intellectually disabled children include a full-blown smile (which may or may not accompany inappropriate laughter), stiffening, and non-cooperation (Hadden 2002). Other observed behaviors include the freezing phenomenon, in which the child acutely feels the pain, and he abruptly pauses without moving his face for several seconds. Look also for episodes of unexplained pallor, diaphoresis, breath-holding, and shrill vocalizations. The FLACC has been revised (r-FLACC) for children with cognitive impairment and appears to be reliable for acute care (Malviya 2006).
The most distressing and perplexing presentation is the parent who brings his or her child with cognitive impairment for “fussiness”, “irritability”, or “I think he’s in pain”. Often, this is after significant investigations have been performed, sometimes repeatedly. Poorly controlled spasticity is an often under-appreciated cause of unexplained pain; treat not with opioids, but with GABA-receptor agonists, such as baclofen or benzodiazepines.
Take special precautions in the administration of opioids or benzodiazepines in children with metabolic disorders (e.g. mitochondrial disease) or various syndromes (e.g. Trisomy 21). They may have a disproportionate reaction to the medication. Start with a low dose in these children and reassess frequently, titrating in small aliquots as needed.
After careful, meticulous investigation in the ED to rule out occult infection, trauma, electrolyte imbalance, or surgical causes, the child with cognitive impairment who continues to be symptomatic despite ED treatment may be admitted for observation. However, in some cases, the addition of gabapentin to the typical regimen has been shown to manage unexplained irritability in these children (Hauer 2007) by treating visceral hyperalgesia.
Multi-trauma
The child with multi-trauma is in need of meticulous critical care. Frequent assessments of pain analgesic response (typically via the intravenous route) are necessary to gauge the child’s trajectory. Unexplained tachycardia may be the early signs of shock. Without controlling the child’s pain, it is difficult to distinguish the extreme tachycardia from pain or from blood loss. If intubated, control the pain first with a fentanyl drip, then use a sedative in addition as needed to keep him comfortable.
The child under palliative care
Children undergoing palliative care require a multidisciplinary approach. This includes engaging the patient’s car team as well as “treating” members of the patient’s family. Examples include the natural course of devastating chromosomal, neurologic, and other congenital conditions; terminal cancer; and trauma, among others (Michelson 2007). Family dynamics and family members’ needs are often overlooked; the family as a whole must be considered. Focus on the productive and beneficial treatments that can be offered. Treat pain promptly, but speak with the parents about end-of-life goals as early as possible, as any analgesic or sedative may have an untoward effect. You do not want to be caught in the position of potentially precipitously providing cardiopulmonary resuscitation in a child undergoing palliative care, because of a lack of understanding of how increasingly large doses of pain medications can affect breathing and circulation (AAP 2000).
Children with ongoing opioid requirements may present not so much with an exacerbation of their chronic pain, but a complication of its treatment. Identify, assess and aggressively treat constipation, nausea and vomiting, pruritus, and urinary retention (Friedrichsdorf 2007); treating side-effects of pain management may be just as important for quality of life as treating the pain itself.
PEARLS AND PITFALLS IN PEDIATRIC PAIN
Allow the child to speak for himself whenever possible. After acknowledging the parent’s input, perhaps try “I want to make sure I understand how the pain is for you. Tell me more.”
Engage parents and communicate the plan to them. Elicit their expectations, and give them of preview of what to expect in the ED.
Opioids are meant for pain caused by acute tissue injury, for the briefest period of time feasible. Older school-aged children and adolescents are increasingly at risk for opioid dependence and addiction.
Premature infants present a challenge in pain control. Their pain is under-recognized, as they often display atypical responses to painful stimuli. Treatment is equally difficult, as they are particularly sensitive to analgesia-sedation. This is important, as this group is even more likely to undergo painful procedures due to their higher-risk status.
Give detailed advice on how to manage pain at home. Set expectations. Let them know you understand and will help them through your good advice that will carry them through this difficult time. Patients and families often just need a plan. Map it out clearly.
SUMMARY
- In pediatric acute pain, know the child; know the family; and know the physiology.
- Use your observational skills enhanced with collateral information to assess and reassess for pain in children.
- Treat pediatric pain well and often. Failure to address the child’s pain has long-lasting consequences.
- Non-pharmacologic treatments for all, pharmacologic treatments for many. A multi-modal approach is the most effective.
- Neonates, infants and toddlers, and school-aged children and adolescents exhibit specific physiology in expression of pain and in response to treatment. Tailor your regimen to your young patient’s physiologic pitfalls and needs.
References
Howard RF. Current status of pain management in children. JAMA. 2003 Nov 12;290(18):2464-9.
Pasero C, McCaffery M. Pain Assessment and Pharmacologic Management. St. Louis, Mo: Mosby; 2011.
Piaget J. Judgment and reasoning in the child. Harcourt & Brace. Oxford, England. 1928.
This post and podcast are dedicated to Sergey M. Motov, MD, FAAEM, for his integrity, hard-won expertise, humility, and innovation. Thank you for making us better doctors, Sergey, and for getting us ever closer to a pain-free ED.
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP