Categories
Abdominal pain is common; so are strongly held myths and legends about what is concerning, and what is not.
One of our largest responsibilities in the Emergency Department is sorting out benign from surgical or medical causes of abdominal pain. Morbidity and mortality varies by age and condition.
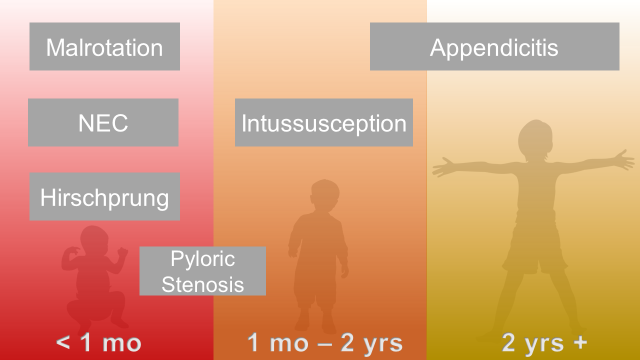
Abdominal Surgical Emergencies in Children: A Relative Timeline
General Advice
Neonate (birth to one month)
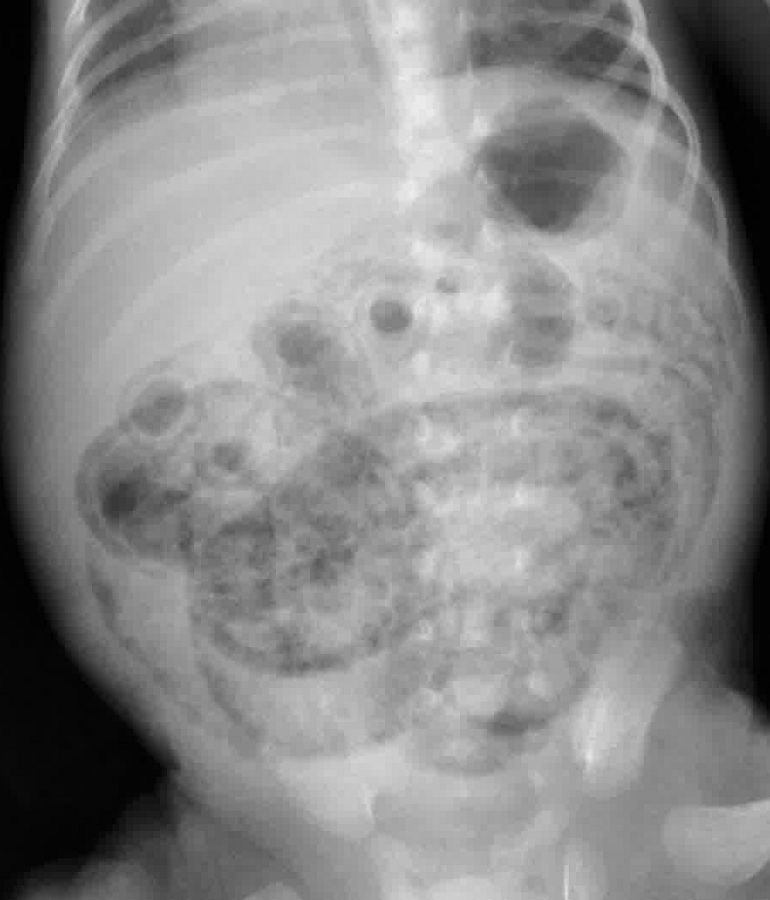
Necrotizing Enterocolitis
Essentials:
- Typically presents in 1st week of life (case reports to 6 months in chronically ill children)
- Extend suspicion longer in NICU graduates
- Up to 10% of all cases of necrotizing enterocolitis are in full-term children
- Pathophysiology is unknown, but likely a translocation of bacteria
Diagnosis:
- Feeding intolerance, abdominal distention
- Abdominal XR: pneumatosis intestinalis
Management:
- IV access, NG tube, broad-spectrum antibiotics, surgery consult, ICU admission
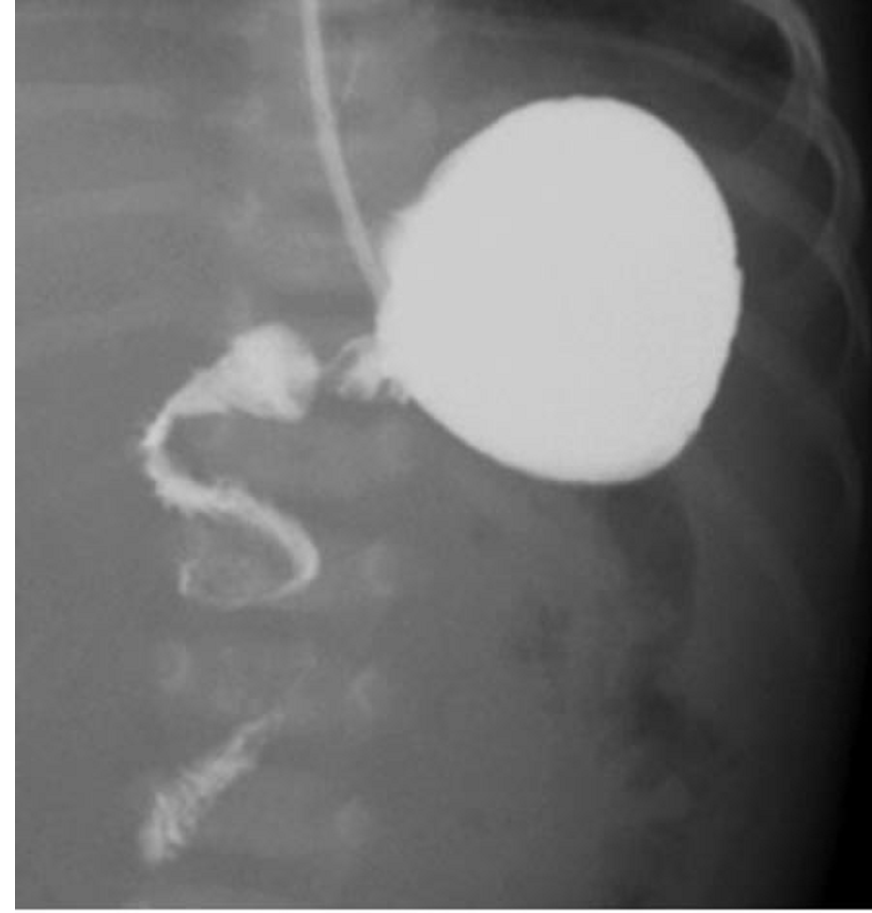
Intestinal Malrotation with Volvulus
Essentials:
- Bilious vomiting (80-100%) in the 1st month; especially in the 1st week
- May look well initially, then rapidly present in shock
- Ladd’s bands: abnormally high tethering of cecum to abdominal wall; peristalsis, volvulus, ischemia
Diagnosis:
- History of bilious emesis is sufficient to involve surgeons
- Upper GI series: corkscrew appearance
- US (if ordered) may show abnormal orientation of and/or flow to superior mesenteric artery and vein
Management:
- Stat surgical consult
- IV access, resuscitation, NG tube to decompress (bowel wall perfusion at risk, distention worsens)
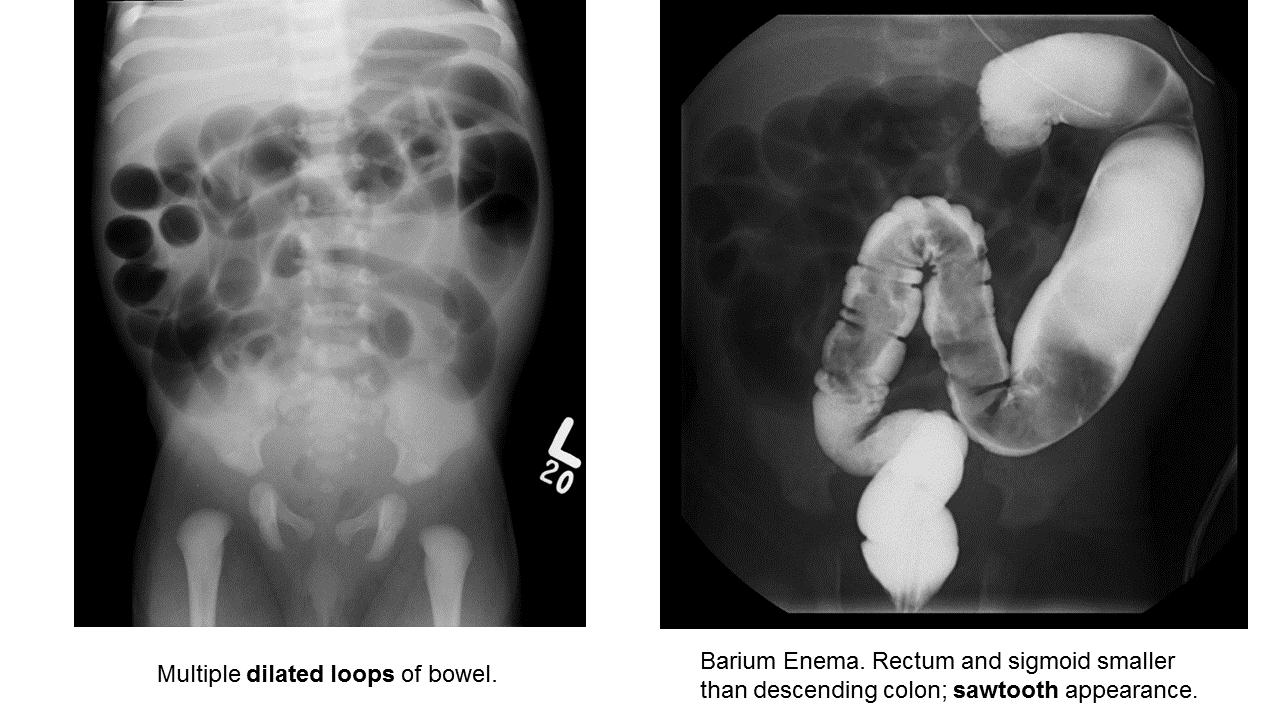
Hirschprung Disease
Essentials:
- Problem in migration of neural crest cells
- Aganglionic colon (80% rectosigmoid; 15-20% proximal to sigmoid; 5% total colonic aganglionosis) colon (known as short-segment disease)
- Poor to no peristalsis: constipation, perforation, and/or sepsis
Diagnosis:
- May be diagnosed early as “failure to pass meconium in 1st 48 hours”
- In ED, presents as either bowel obstruction or enterocolitis
- Contrast enema
- Beware of the toxic megacolon (vomiting, distention, sepsis)
Management:
- Resuscitation, antibiotics, NG tube decompression, surgical consultation; stable patients may need rectal biopsy for confirmation
- Staged surgery (abdominoperineal pull-through with diverting colostomy, subsequent anastomosis) versus one-stage repair.
Infant and Toddler (1 month to 2 years)
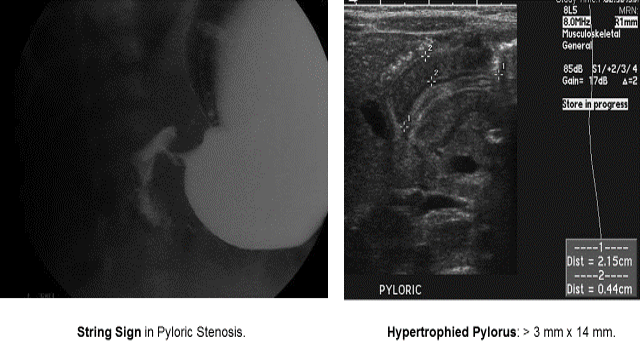
Pyloric Stenosis
Essentials:
- Hypertrophy of pyloric sphincter; genetic, environmental, exposure factorsString Sign in Pyloric Stenosis.
Diagnosis:
- Hungry, hungry, not-so-hippos; they want to eat all of the time, but cannot keep things down
- Poor weight gain (less than 20-30 g/day)
- US: “π–loric stenosis” (3.14); pylorus dimensions > 3 mm x 14 mm
- UGI: “string sign”
Management:
- Trial of medical treatment with oral atropine via NGT (muscarinic effects decrease pyloric tone)
- Ramstedt pyloromyotomy (definitive)
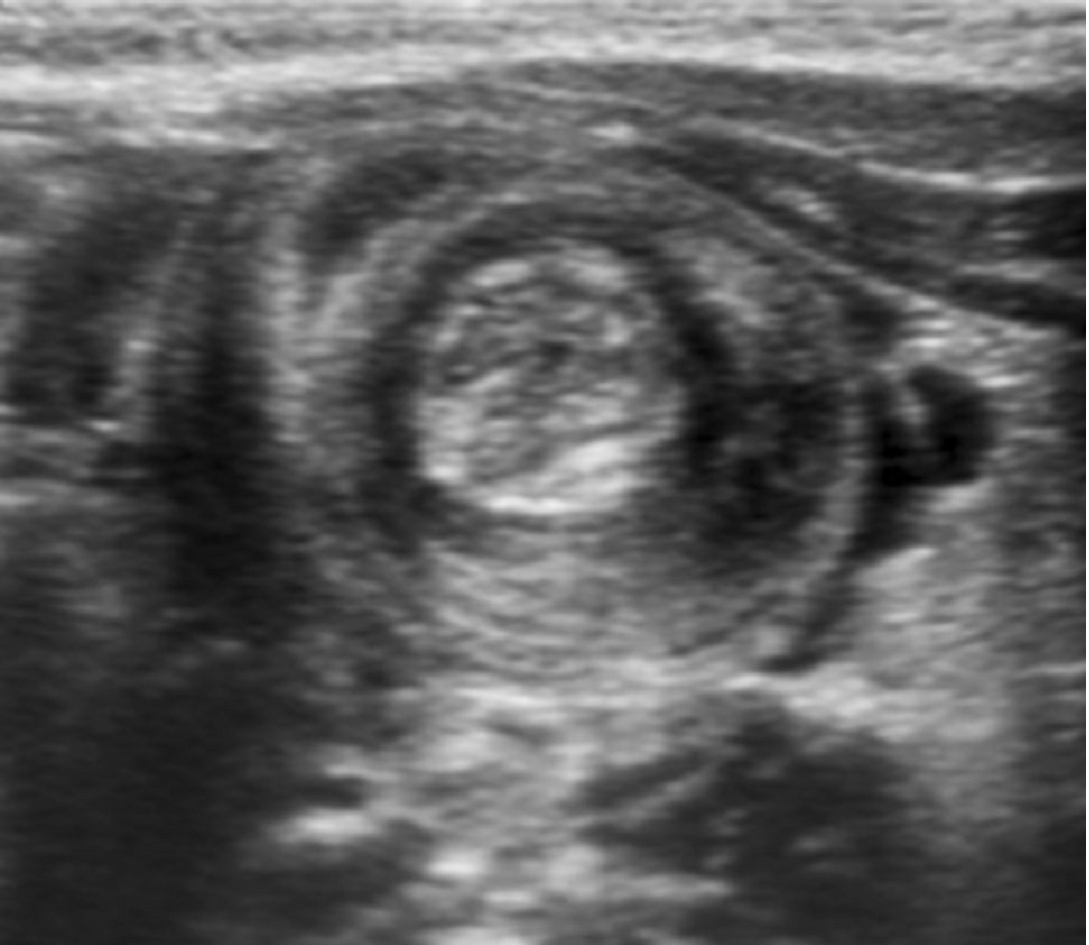
Intussusception
Essentials:
- Majority (90%) ileocolic; no pathological lead point
- Small minority (4%) ileoileocolic due to lead point: Meckel’s diverticulum, polyp, Peyer’s patches, Henoch-Schönlein purpura (intestinal hematoma)
Diagnosis:
- Ultrasound sensitivity and specificity near 100% in experienced hands
- Abdominal XR may show non-specific signs; used mainly to screen for perforation before reduction
Management:
- Hydrostatic enema: contrast (barium or water-soluble contrast with fluoroscopy) or saline (with ultrasound)
- Air-contrast enema: air or carbon dioxide (with either fluoroscopy or ultrasound); higher risk for perforation than hydrostatic (1% risk), but generally safer than perforation from contrast
- Consider involving surgical service early (precaution before reduction)
- Traditional disposition is admission; controversial: home discharge from ED
Young Child and Older (2 years and up)
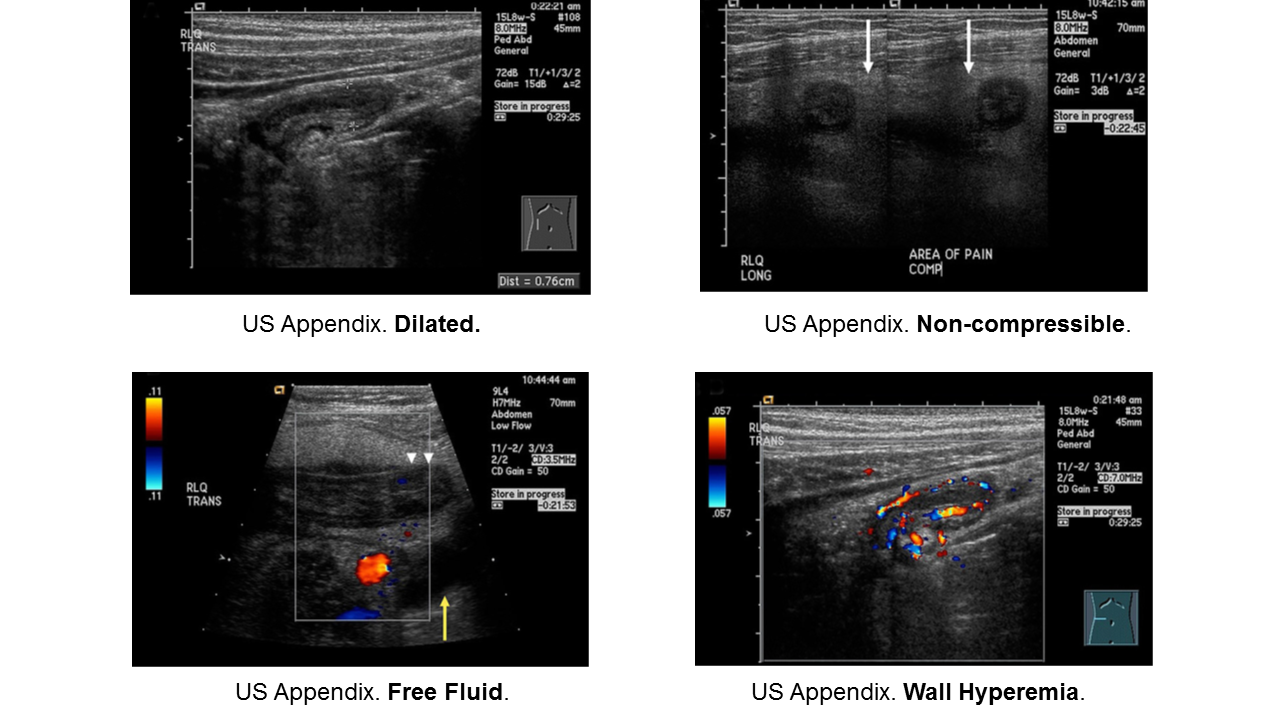
Appendicitis
Essentials:
- Appendicitis occurs in all ages, but rarer in infants. Infants do not have fecalith; rather they have some other anatomic or congenital condition.
- More common in school-aged children (5-12 years) and adolescents
- Younger children present atypically, more likely to have perforated when diagnosed.
Diagnosis:
- Non-specific signs and symptoms
- Often have abdominal pain first; vomiting comes later
- Location/orientation of appendix varies
- Appendicitis scores vary in their performance
- Respect fever and abdominal pain
Management:
- Traditional: surgical
- On the horizon: identification of low-risk children who may benefit from trial of antibiotics
- If perforated, interval appendectomy (IV antibiotics via PICC for 4-6 weeks, then surgery)
Obstruction
Essentials:
- Same pathophysiology and epidemiology as adults: “ABC” – adhesions, “bulges” (hernias), and cancer.
Diagnosis:
- Obstruction is a sign of another condition. Look for cause of obstruction: surgical versus medical
- Abdominal XR in low pre-test probability
- CT abdomen/pelvis for moderate-to-high risk; confirmation and/or surgical planning
Management:
- Treat underlying cause
- NG tube to low intermittent wall suction
- Admission, fluid management, serial examinations
Take these pearls home:
- Consider surgical pathology early in encounter
- Resuscitate while you investigate
- Have a low threshold for imaging and/or consultation, especially in preverbal children
Selected References
Necrotizing Enterocolitis
Neu J, Walker A. Necrotizing Enterocolitis. N Eng J Med. 2011; 364(3):255-264.
Niño DF et al. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nature. 2016; 13:590-600.
Walsh MC et al. Necrotizing Enterocolitis: A Practitioner’s Perspective. Pediatr Rev. 1988; 9(7):219-226.
Malrotation with Midgut Volvulus
Applegate KE. Intestinal Malrotation in Children: A Problem-Solving Approach to the Upper Gastrointestinal Series. Radiographics. 2006; 26:1485-1500.
Kapfer SA, Rappold JF. Intestinal Malrotation – Not Just the Pediatric Surgeon’s Problem. J Am Coll Surg. 2004; 199(4):628-635.
Lee HC et al. Intestinal Malrotation and Catastrophic Volvulus in Infancy. J Emerg Med. 2012; 43(1):49-51.
Martin V, Shaw-Smith C. Review of genetic factors in intestinal malrotation. Pediatr Surg Int. 2010; 26:769-781.
Nehra D, Goldstein AM. Intestinal malrotation: Varied clinical presentation from infancy through adulthood. Surgery. 2010; 149(3):386-391.
Hirschprung Disease
Amiel J, Sproat-Emison E, Garcia-Barcelo M, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet 2008; 45:1.
Arshad A, Powell C, Tighe MP. Hirschsprung's disease. BMJ 2012; 345:e5521.
Aworanti OM, McDowell DT, Martin IM, Quinn F. Does Functional Outcome Improve with Time Postsurgery for Hirschsprung Disease? Eur J Pediatr Surg 2016; 26:192.
Clark DA. Times of first void and first stool in 500 newborns. Pediatrics 1977; 60:457.
Dasgupta R, Langer JC. Evaluation and management of persistent problems after surgery for Hirschsprung disease in a child. J Pediatr Gastroenterol Nutr 2008; 46:13.
De Lorijn F, Reitsma JB, Voskuijl WP, et al. Diagnosis of Hirschsprung's disease: a prospective, comparative accuracy study of common tests. J Pediatr 2005; 146:787.
Doig CM. Hirschsprung's disease and mimicking conditions. Dig Dis 1994; 12:106.
Khan AR, Vujanic GM, Huddart S. The constipated child: how likely is Hirschsprung's disease? Pediatr Surg Int 2003; 19:439.
Singh SJ, Croaker GD, Manglick P, et al. Hirschsprung's disease: the Australian Paediatric Surveillance Unit's experience. Pediatr Surg Int 2003; 19:247.
Suita S, Taguchi T, Ieiri S, Nakatsuji T. Hirschsprung's disease in Japan: analysis of 3852 patients based on a nationwide survey in 30 years. J Pediatr Surg 2005; 40:197.
Sulkowski JP, Cooper JN, Congeni A, et al. Single-stage versus multi-stage pull-through for Hirschsprung's disease: practice trends and outcomes in infants. J Pediatr Surg 2014; 49:1619.
Pyloric Stenosis
Aspelund G, Langer JC. Current management of hypertrophic pyloric stenosis. Semin Pedaitr Surg. 2007; 16:27-33.
Dias SC et al. Hypertrophic pyloric stenosis: tips and tricks for ultrasound diagnosis. Insights Imaging. 2012; 3:247-250.
Kawahara H et al. Medical treatment of infantile hypertrophic pyloric stenosis: should we always slice the olive? J Pediatr Surg. 2005; 40:1848-1851.
Mack HC. Adult Hypertrophic Pyloric Stenosis. Arch Inter Med. 1959; 104:78-83.
Meissner PE et al. Conservative treatment of infantile hypertrophic pyloric stenosis with intravenous atropine sulfate does not replace pyloromyotomy. Pediatr Surg Int. 2006; 22:1021-1024.
Mercer AE, Phillips R. Can a conservative approach to the treatment of hypertrophic pyloric stenosis with atropine be considered a real alternative to pyloromyotomy? Arch Dis Child. 2013; 95(6): 474-477.
Pandya S, Heiss K, Pyloric Stenosis in Pediatric Surgery.Surg Clin N Am. 2012; 92:527-39.
Peters B et al. Advances in infantile hypertrophic pyloric stenosis. Expert Rev Gastroenterol Hepatol. 2014; 8(5):533-541.
Intussusception
Apelt N et al. Laparoscopic treatment of intussusception in children: A systematic review. J Pediatr Surg. 2013; 48:1789-1793.
Applegate KE. Intussusception in Children: Imaging Choices. Semin Roentgenol. 2008; 15-21.
Bartocci M et al. Intussusception in childhood: role of sonography on diagnosis and treatment. J Ultrasound. 2015; 18 Gilmore AW et al. Management of childhood intussusception after reductiion by enema. Am J Emerg Med. 2011; 29:1136-1140.:205-211.
Chien M et al. Management of the child after enema-reduced intussusception: hospital or home? J Emerg Med. 2013; 44(1):53-57.
Cochran AA et al. Intussusception in traditional pediatric, nontraditional pediatric, and adult patients. Am J Emerg Med. 2011; 523-527.
Loukas M et al. Intussusception: An Anatomical Perspective With Review of the Literature. Clin Anatomy. 2011; 24: 552-561.
Mendez D et al. The diagnostic accuracy of an abdominal radiograph with signs and symptoms of intussusception. Am J Emerg Med. 2012; 30:426-431.
Whitehouse et al. Is it safe to discharge intussusception patients after successful hydrostatic reduction? J Pediatr Surg. 2010; 45:1182-1186.
Appendicitis
Amin P, Chang D. Management of Complicated Appendicitis in the Pediatrc Population: When Surgery Doesn’t Cut it. Semin Intervent Radiol. 2012; 29:231-236
Blakely ML et al. Early vs Interval Appendectomy for Children With Perforated Appendicitis. Arch Surg. 2011; 146(6):660-665.
Bundy DG et al. Does This Child Have Appendicitis? JAMA. 2007; 298(4):438-451.
Cohen B et al. The non-diagnostic ultrasound in appendicitis: is a non-visualized appendix the same as a negative study? J Pediatr Surg. 2015 Jun;50(6):923-7
Herliczek TW et al. Utility of MRI After Inconclusive Ultrasound in Pediatric Patients with Suspected Appendicitis. AJT. 2013; 200:969-973.
Janitz et al. Ultrasound Evaluation for Appendicitis. J Am Osteopath Coll Radiol. 2016; 5(1):5-12.
Kanona H et al. Stump Appendicitis: A Review. Int J Surg. 2012; 10:4255-428.
Kao LS et al. Antibiotics vs Appendectomy for Uncomplicated Acute Appendicitis. Evid Based Rev Surg. 2013;216(3):501-505.
Petroianu A. Diagnosis of acute appendicitis. Int J Surg. 2012; 10:115-119.
Mazeh H et al. Tip appendicitis: clinical implications and management. Amer J Surg. 2009; 197:211-215.
Puig S et al. Imaging of Appendicitis in Children and Adolescents. Semin Roentgenol. 2008; 22-28.
Schizas AMP, Williams AB. Management of complex appendicitis. Surgery. 2010; 28(11):544-548.
Shogilev DJ et al. Diagnosing Appendicitis: Evidence-Based Review. West J Emerg Med. 2014; 15(4):859-871.
Wray CJ et al. Acute Appendicitis: Controversies in Diagnosis and Management. Current Problems in Surgery. 2013; 50:54-86
Intestinal Obstruction
Babl FE et al. Does nebulized lidocaine reduce the pain and distress of nasogastric tube insertion in young children? A randomized, double-blind, placebo-controlled trial. Pediatrics. 2009 Jun;123(6):1548-55
Chinn WM, Zavala DC, Ambre J. Plasma levels of lidocaine following nebulized aerosol administration. Chest 1977;71(3):346-8.
Cullen L et al. Nebulized lidocaine decreases the discomfort of nasogastric tube insertion: a randomized, double-blind trial. Ann Emerg Med. 2004 Aug;44(2):131-7.
Gangopadhyay AN, Wardhan H. Intestinal obstruction in children in India. Pediatr Surg Int. 1989; 4:84-87.
Hajivassiliou CA. Intestinal Obstruction in Neonatal/Pediatric Surgery. Semin Pediatr Surg. 2003; 12(4):241-253.
Hazra NK et al. Acute Intestinal Obstruction in children: Experience in a Tertiary Care Hospital. Am J Pub Health Res. 2015; 3(5):53-56.
Kuo YW et al. Reducing the pain of nasogastric tube intubation with nebulized and atomized lidocaine: a systematic review and meta-analysis. J Pain Symptom Manage. 2010 Oct;40(4):613-20. .
Pediatric Surgery
Irish MS et al. The Approach to Common Abdominal Diagnoses in Infants and Children. Pedaitr Clin N Am. 1998; 45(4):729-770.
Louie JP. Essential Diagnosis of Abdominal Emergencies in the First Year of Life. Emerg Med Clin N Am. 2007; 25:1009-1040.
McCullough M, Sharieff GQ. Abdominal surgical emergencies in infants and young children. Emerg Med Clin N Am. 2003; 21:909-935.
Pepper VK et al. Diagnosis and Management of Pediatric Appendicitis, Intussusception, and Meckel Diverticulum. Surg Clin N Am. 2012
This post and podcast are dedicated to Mr Ross Fisher for his passion and spirit of collaboration in all things #FOAMed. Thank you, sir!
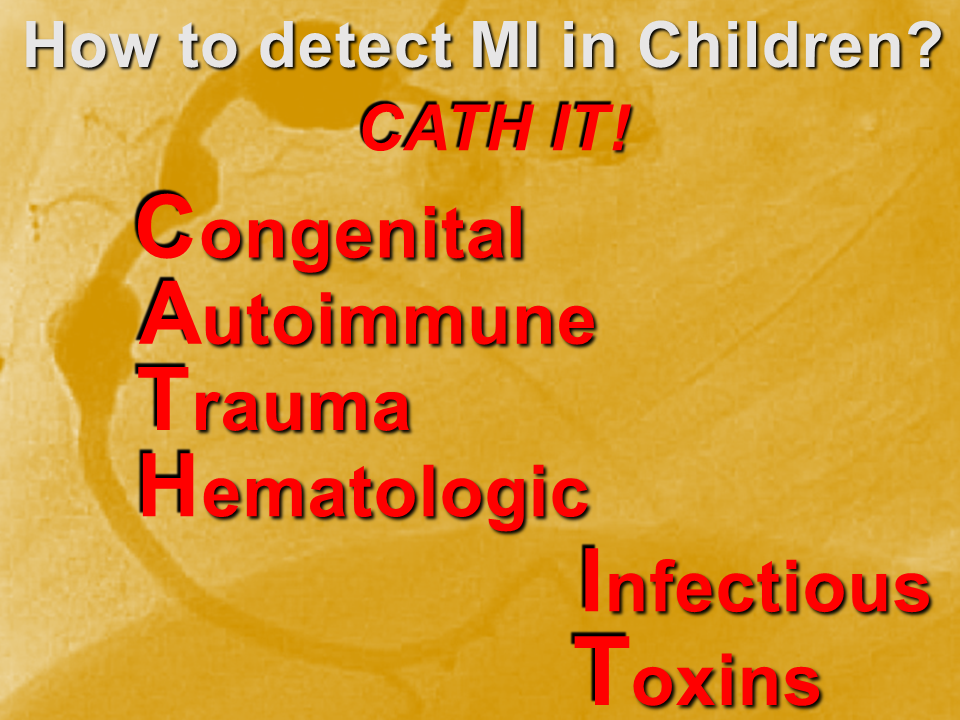
Myocardial infarction (MI) in children is uncommon, but underdiagnosed. This is due to two main factors: the etiologies are varied; and the presenting symptoms are “atypical”.
We need a mental metal detector!
Case examples
Congenital
Two main presentations of MI due to congenital lesions: novel and known. The novel presentation is at risk for underdiagnosis, due to its uncommonness and vague, atypical symptoms. There are usually some red flags with a careful H&P. The known presentation is a child with a history of congenital heart disease, addressed by corrective or palliative surgery. This child is at risk for expected complications, as well as overdiagnosis and iatrogenia. Risk stratify, collaborate with specialists.
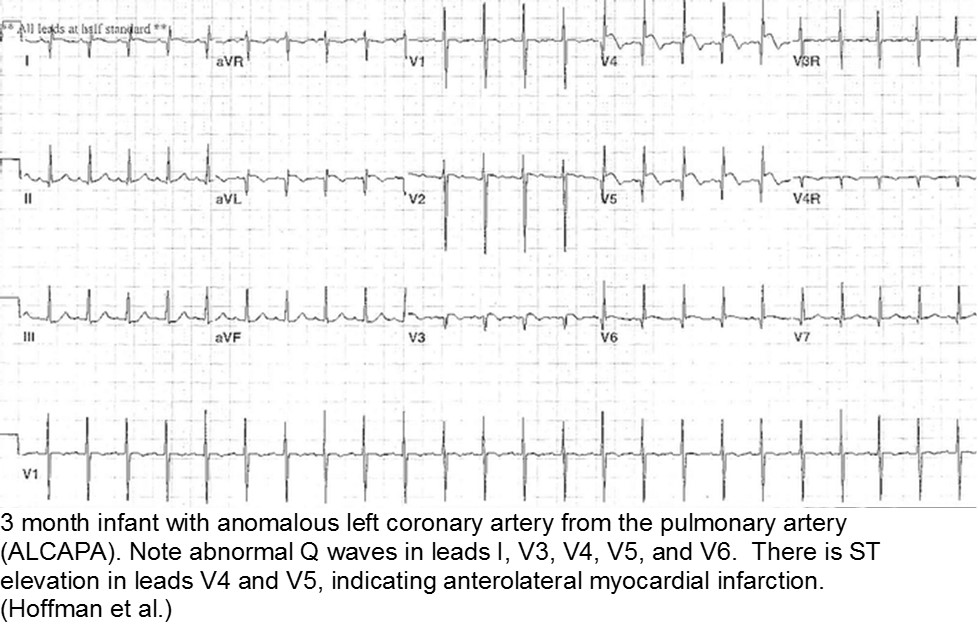
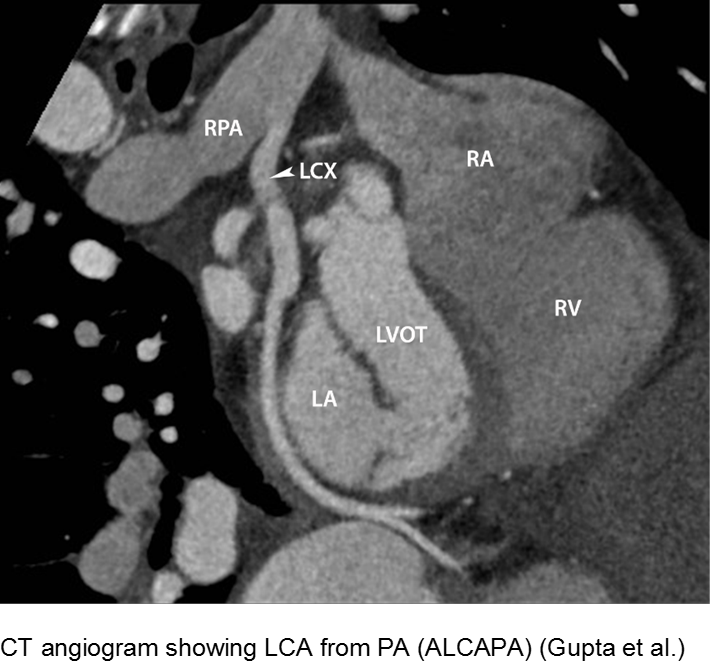
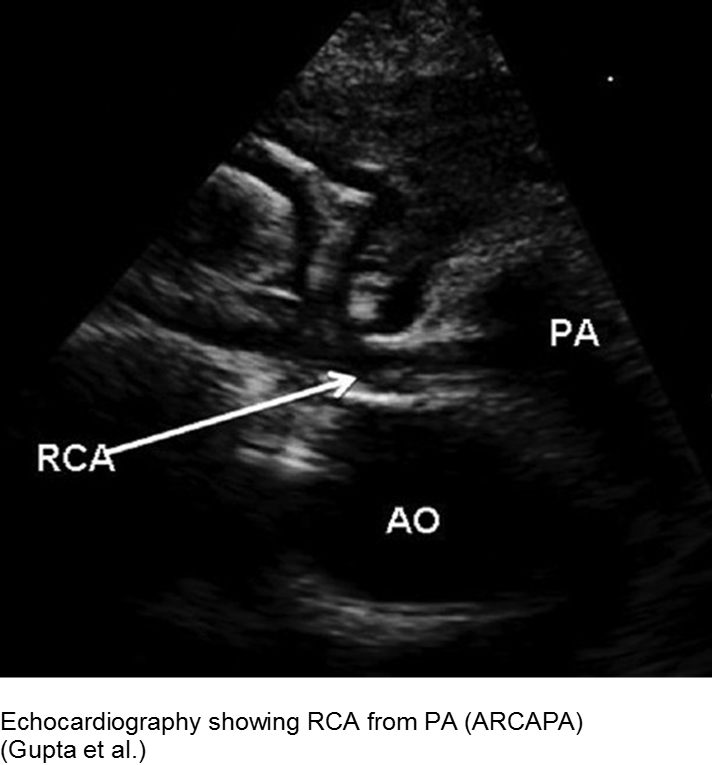
The fussy, sweaty feeder: ALCAPA
Anomalous Left Coronary Artery from the Pulmonary Artery (ALCAPA) is an example of what can go wrong during fetal development: any abnormality in the number, origin, course, or morphology of the coronary arteries can present as a neonate with sweating during feeds (steal syndrome), an infant in CHF, or an older child with failure to thrive or poor exercise tolerance.
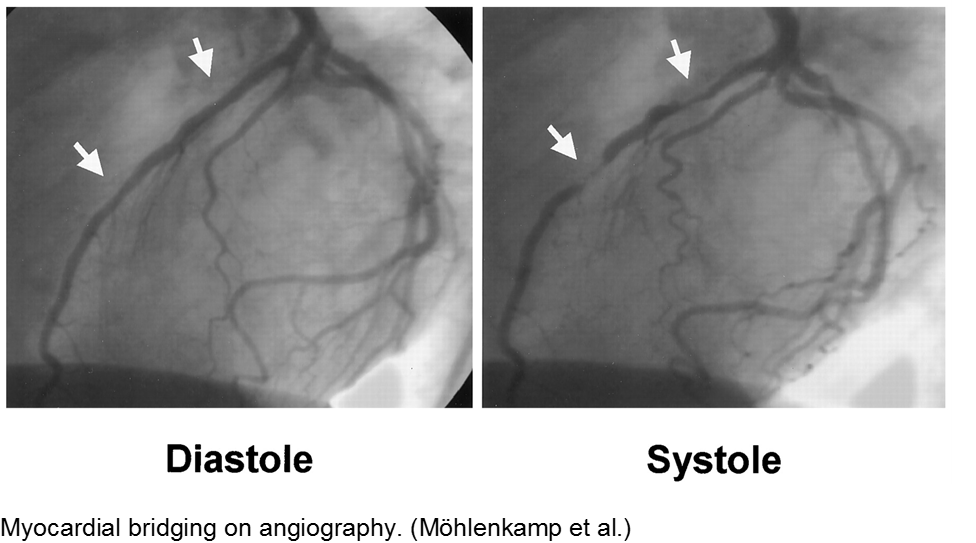
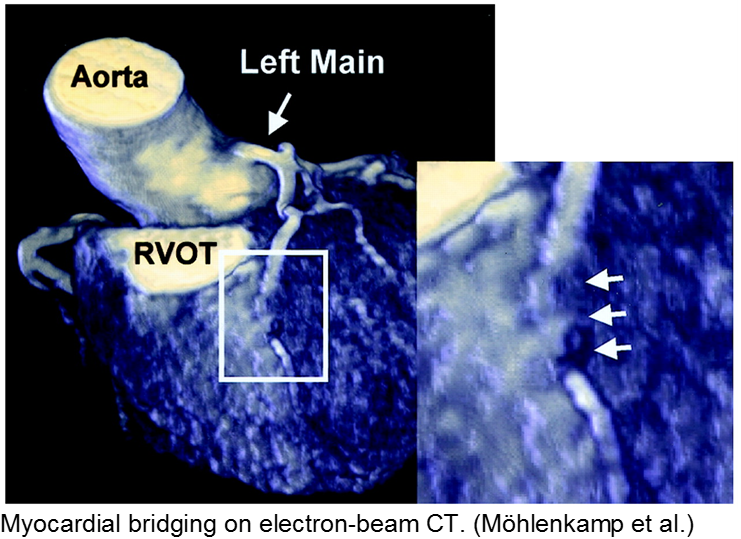
The stable child with chest pain: myocardial bridge
Normal coronary arteries run along the epicardial surface of the heart, with projections into the myocardium. If part of the artery’s course runs within the myocardium (i.e. the artery weaves into and/or out of the myocardium), then there is a myocardial bridge of the coronary artery. With every systolic contraction, the artery is occluded.
Although a myocardial bridge may not cause symptoms (especially at distal portions), the area it supplies is at risk.
With any minor trauma or exertion, demand may outpace supply, resulting in ischemia.
Diagnosis is made on coronary angiography.
The unwell child post-cardiac surgery: Fontan problems
The child with single ventricle physiology may have a Norwood procedure at birth (creation of a neoaorta, atrial septectomy, and Blalock-Taussig shunt), a Bidirectional Glenn procedure at 3-6 months (shunt removed, superior vena cava connected to pulmonary arteries), and a Fontan procedure at about 2-3 years of age (inferior vena cava blood flow is shunted into the pulmonary arteries).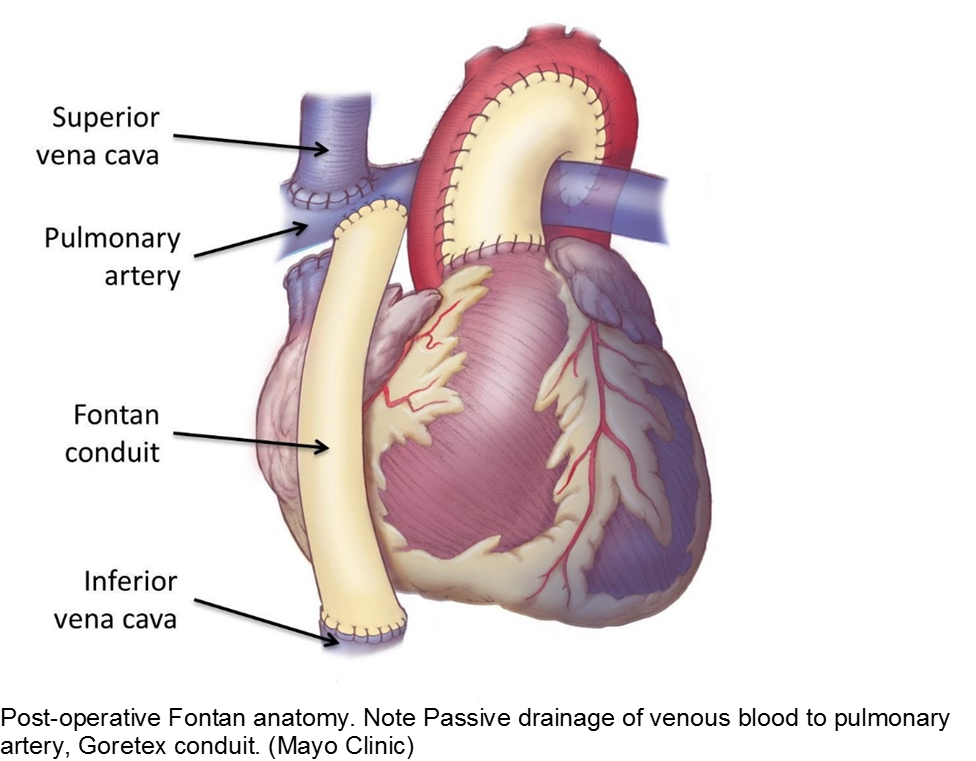
These children depend on their preload to run blood passively into the pulmonary circuit; afterload reduction is also important to compensate for a poor left ejection fraction, as well as to avoid the development of pulmonary hypertension. They are typically on an anticoagulant (often aspirin), a diuretic (e.g. furosemide), and an afterload reduction agent (e.g. enalapril).
Any disturbance in volume status (hyper- or hypovolemia), anticoagulation, or afterload may cause myocardial strain or infarction. Take the child s/p Fontan seriously and involve his specialists early with any concerns.
Autoimmune
The body’s inflammatory-mediated reaction to a real or perceived insult can cause short- and long-term cardiac sequelae. Find out how well the underlying disease is controlled, and what complications the child has had in the past.
The red, hot, crispy, flaky child: acute Kawasaki disease
Kawasaki disease (KD) is an acute systemic vasculitis, diagnosed by the presence of fever for five or more days accompanied by four or more criteria: bilateral conjunctival injection, mucositis, cervical lymphadenopathy, polymorphous rash, and palmar or sole desquamation. The criteria may occur (and disappear) at any time during the illness.
Infants are under double jeopardy with Kawasaki Disease. They are more likely to have incomplete KD (i.e. not fulfill strict criteria) and if they have KD, they are more likely to suffer the dangerous consequences of aneurysm formation (chiefly coronary arteries, but also brain, kidney). Have a low threshold for investigation.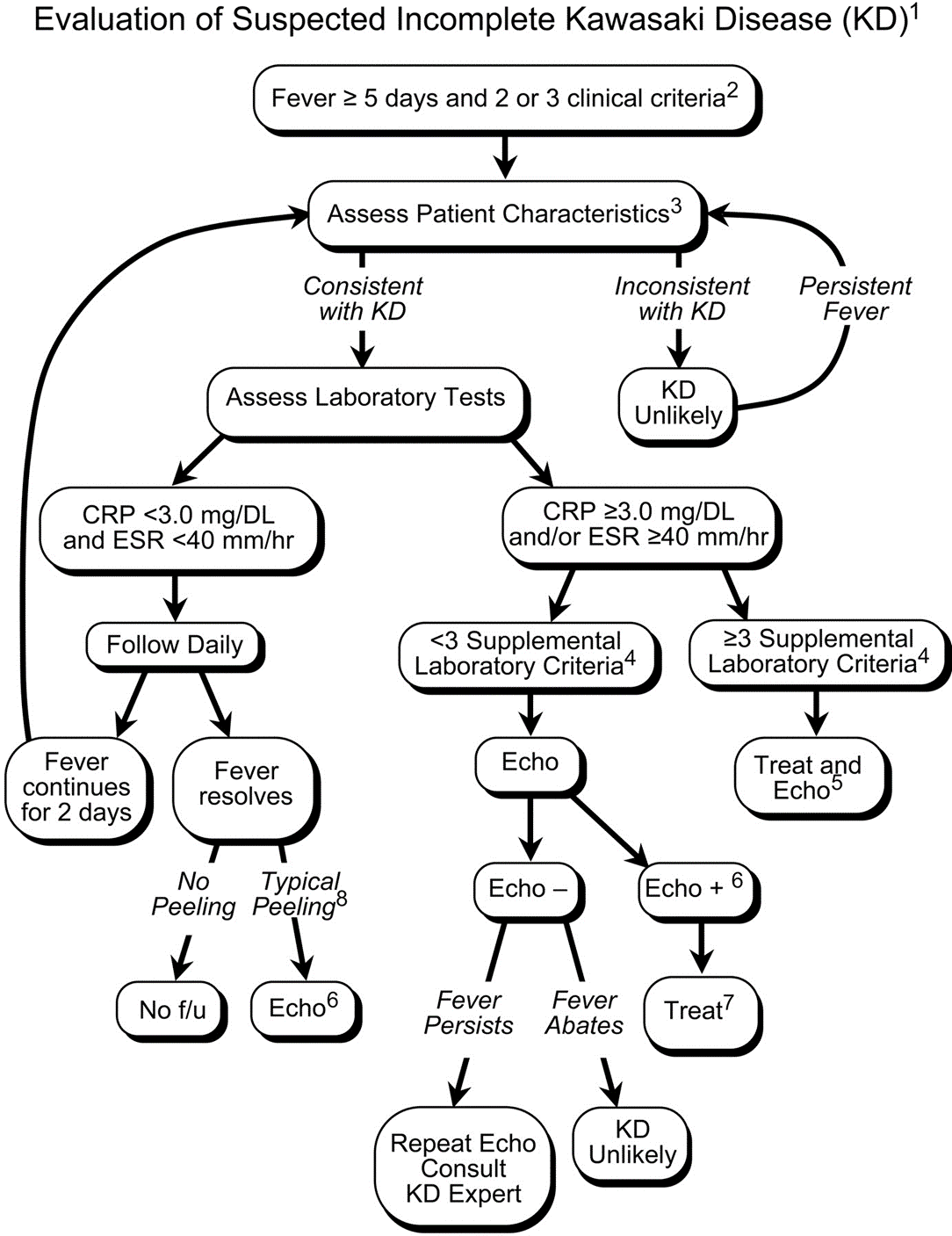
Treatment includes 2 g/kg/day IVIG and high-dose aspirin (30-50 mg/kg/day) acutely, then low-dose aspirin (5 mg/kg/day) for weeks to months. Regular and long-term follow-up with Cardiology is required.
The aftermath: sequelae of Kawasaki disease
The family and child with a history of KD may have psychological trauma and continuous anxiety about the child’s risk of MI. Approximately 4.7% of children who were promptly diagnosed and correctly treated will go on to have cardiac sequelae.
Children who have no detected cardiac sequelae by 8 weeks, typically continue to be asymptomatic up to 20 years later.
Smaller aneurysms tend to regress over time, especially those < 6 mm.
Thrombi may calcify, or the lumen may become stenotic due to myofibroblast proliferation. Children with any coronary artery dilatation from KD should be followed indefinitely.
Giant aneurysms (≥8 mm) connote the highest risk for MI.
Parents often are concerned about recurrence, and any subsequent fever can be distressing. There is a low rate of recurrence for KD: approximately 2%. Infants who have coronary aneurysms are at the highest risk for recurrence.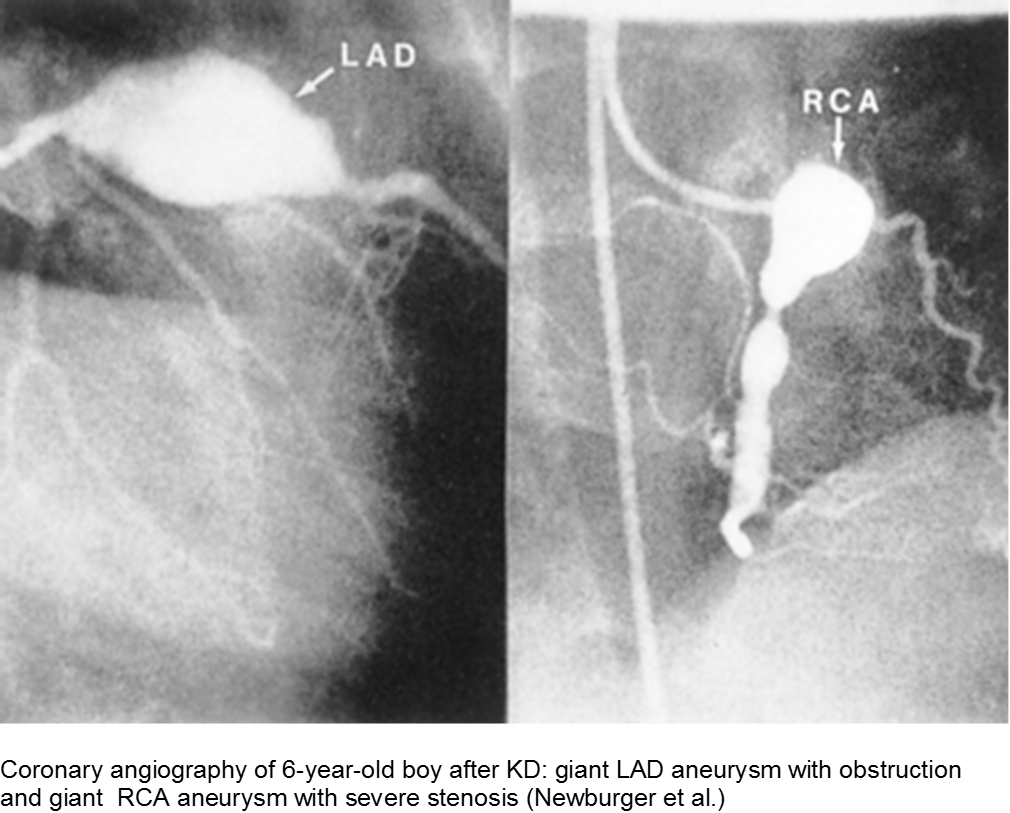
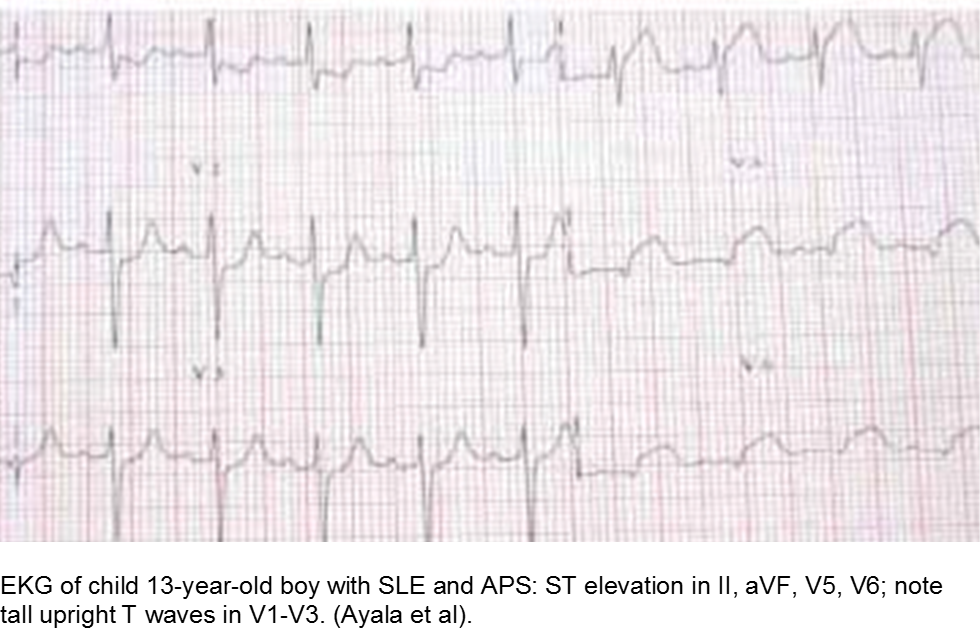
The older child with vague chest complaints and hypercoagulability: Systemic Lupus Erythematosus and Anti-Phospholipid Syndrome
Up to 15% of cases of SLE begin in childhood. Adult criteria are used, with the caveat that the diagnosis of SLE in children can be challenging; many children only manifest a few of the criteria initially before going on to develop further systemic involvement.
The Systemic Lupus International Collaborating Clinics (SLICC) revised the criteria in 2012. The patient should have ≥4/17 clinical and/or immunologic criteria. The clinical criteria are: acute cutaneous (malar); chronic cutaneous (discoid); oral; alopecia; synovitis; serositis; renal; neurologic; hemolytic anemia; leukopenia; or thrombocytopenia. The immunologic criteria are: ANA; anti-dsDNA; anti-Sm; antiphospholipid; low complement; and/or Direct Coombs (in absence of hemolytic anemia). At least one criterion should be clinical, and at least one should be immunologic.
Children with antiphospholipid syndrome (APS) may occur with or without SLE. Patients are at risk for venous and arterial thrombi formation. APS may also cause structural damage, such as valvular thickening and valvular nodes (Libman-Sacks endocarditis). Mitral and aortic valves are at the highest risk.
Although most children with chest pain will not have MI, those with comorbidities should be investigated carefully.
Trauma
Direct, blunt trauma to the chest can cause myocardial stunning, dysrhythmias, or an asymptomatic rise in Troponin I. However, some children are at risk for disproportionate harm due to a previously unknown risk factor. Clinically significant cardiac injury occurs in up to 20% of patients with non-penetrating thoracic trauma.
The motor vehicle collision: blunt myocardial injury
Direct trauma (steering wheel, airbag, seatbelt), especially in fast acceleration-deceleration injury, may cause compression of the heart between the sternum and the thoracic spine.
Electrocardiography (ECG) should be performed on any patient with significant blunt chest injury. A negative ECG is highly consistent with no significant blunt myocardial injury.
Any patient with a new abnormality on ECG (dysrhythmia, heart block, or signs of ischemia) should be admitted for continuous ECG monitoring.
Elevation in troponin is common, but not predicted. A solitary elevated troponin without ECG abnormality is of unclear significance. Author’s advice: obtain troponin testing if there is an abnormal ECG, more than fleeting suspicion of BCI, and/or the child will be admitted for monitoring.
Hemodynamically labile children should be resuscitated and a stat transesophageal echocardiogram obtained.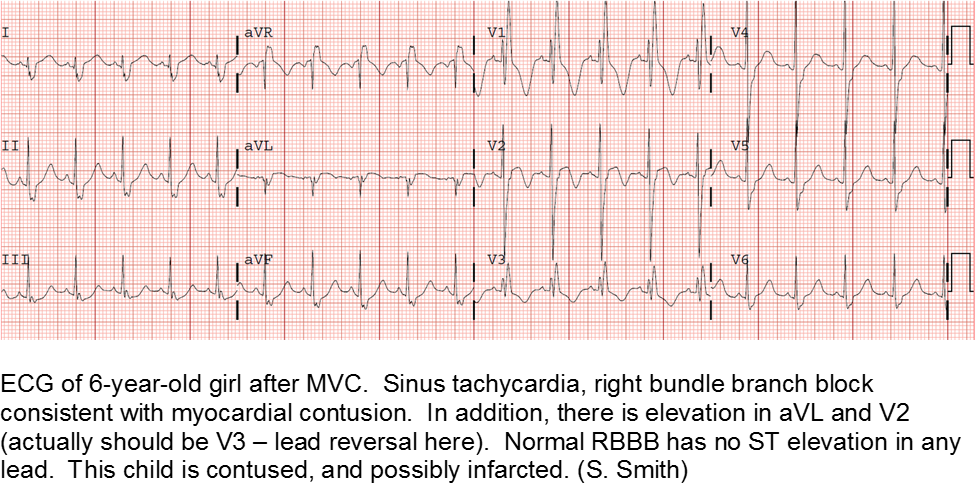
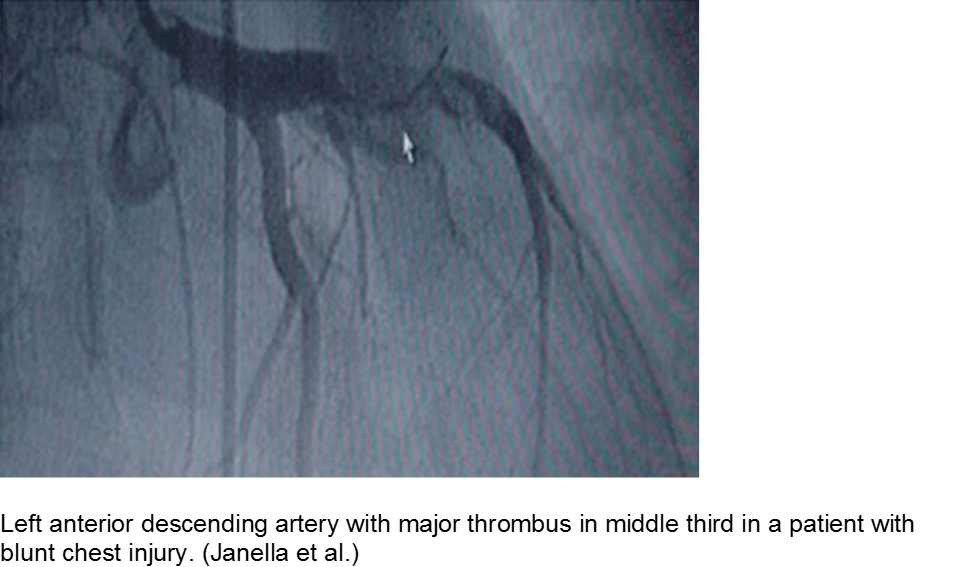
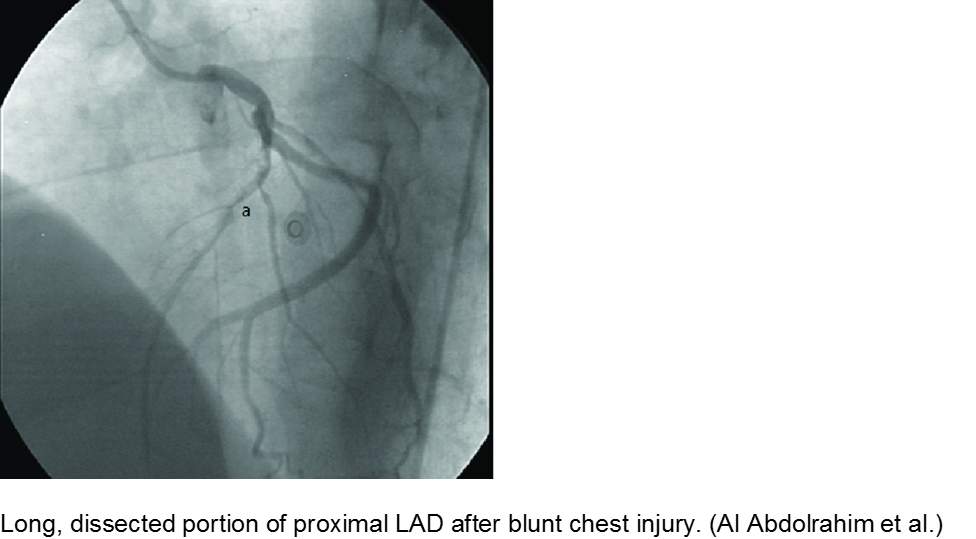
The high-velocity object: coronary artery dissection or thrombus
Direct trauma (e.g. MVC, baseball, high-velocity soccer ball) may cause damage to the left anterior descending artery or left circumflex artery, at the highest risk due to their proximity to the chest wall. Thrombosis and/or dissection may result, often presenting in a focal pattern of ischemia on the ECG.
Echocardiography may reveal valvular damage related to the injury, as well as effusion and ejection fraction. Since there is often a need to investigate the coronary anatomy, percutaneous coronary intervention (PCI) is recommended.
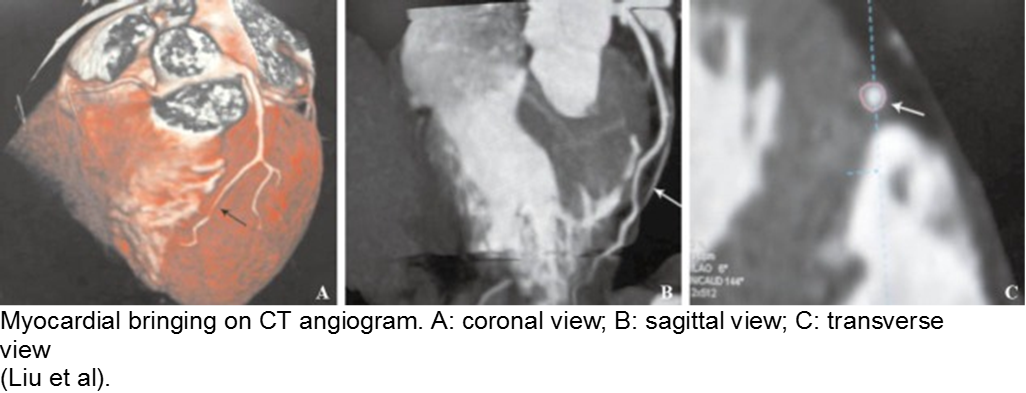
The minor trauma with disproportionate complaint: myocardial bridge
As mentioned in the congenital section (above), a known variation of a coronary artery’s course involves weaving in and out of the myocardium, creating a baseline risk for ischemia. Even minor trauma in a child with a myocardial bridge may cause acute thrombus, or slow stenosis from resulting edema. Unfortunately, the presence of myocardial bridging is often unknown at the time of injury. Approximately 25% of the population may have myocardial bridging, based on autopsy studies. Take the child seriously who has disproportionate symptoms to what should be a minor injury.
Hematologic
Coagulopathic and thrombophilic states may predispose children to focal cardiac ischemia. The best documented cormorbidity is sickle cell disease, although other pro-thrombotic conditions also put the child at risk.
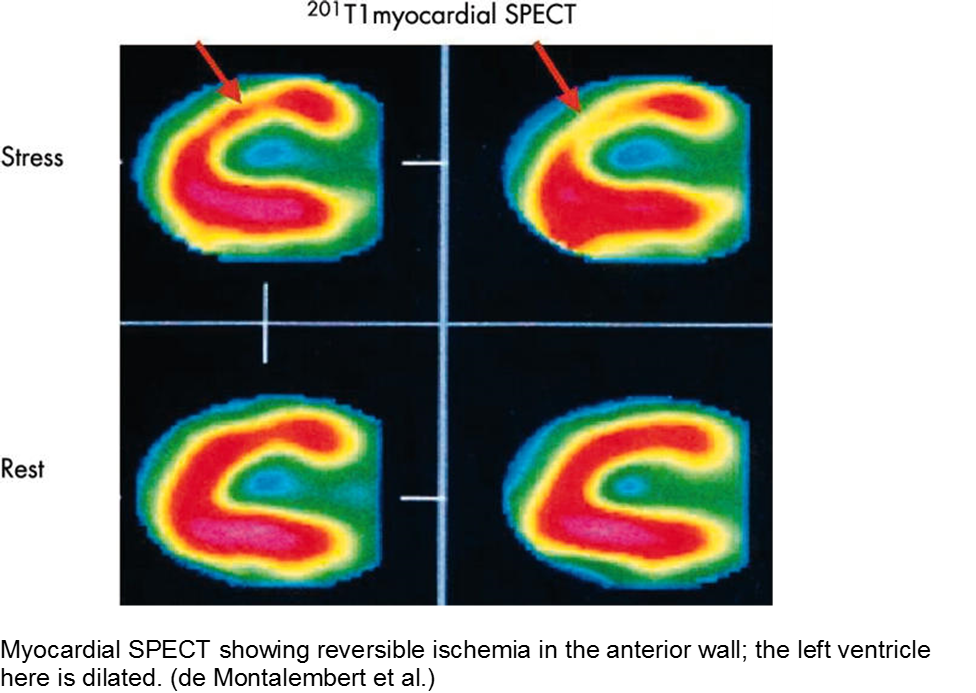
The child with sickle cell disease and chest pain: when it’s not acute chest syndrome
Sickle cell disease (SCD) can affect any organ system, although the heart is traditionally considered a lower-risk target organ for direct sickling and ischemia. The major cardiac morbidity in sickle cell is from strain, high-output failure and multiple, serial increases in myocardial demand, causing left ventricular hypertrophy and congestive heart failure.
However, there is mounting evidence that acute myocardial ischemia in sickle cell disease may be underappreciated and/or attributed to other causes of chest pain.
Other cardiac sequelae from SCD include pulmonary hypertension, left ventricular dysfunction, right ventricular dysfunction, and chronic iron overload.
Evidence of myocardial ischemia/infarction in children with SCD has been demonstrated on single-photon emission computed tomography (SPECT) scan.
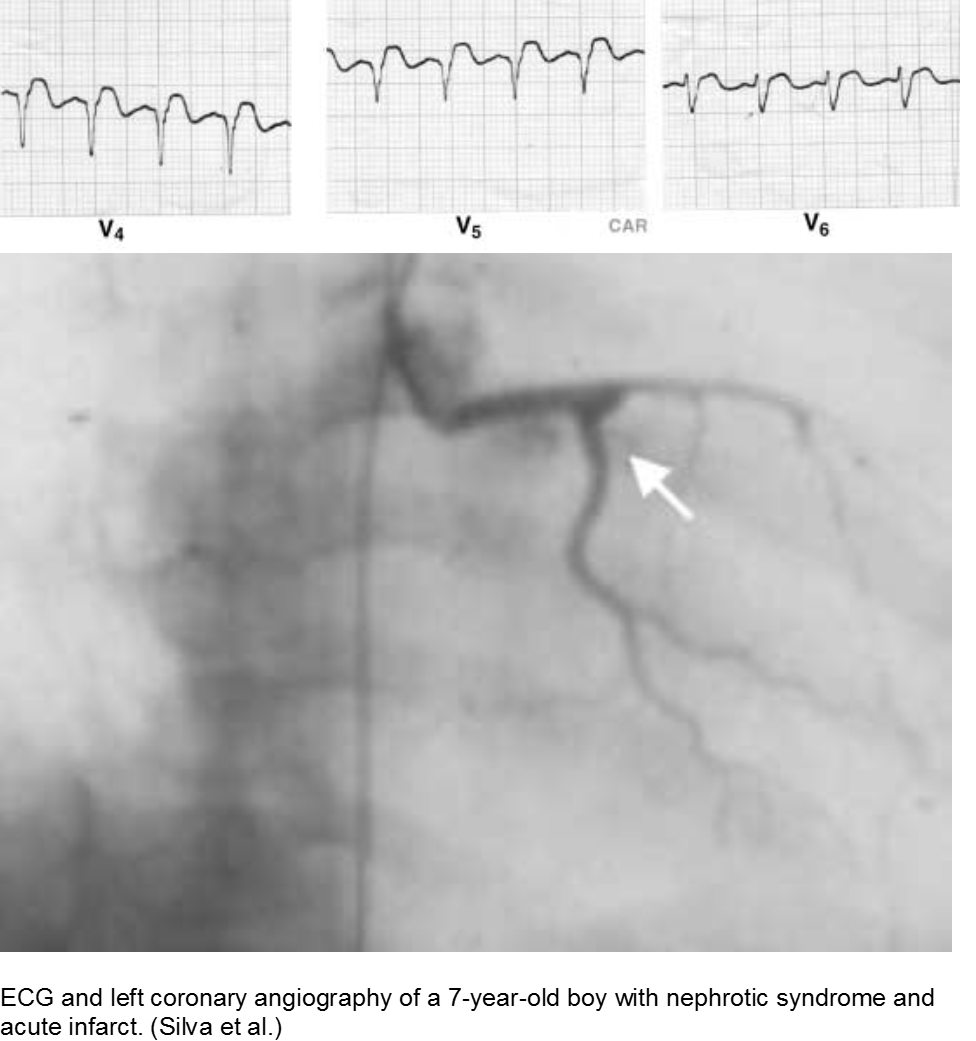
The puffy faced child with chest pain: nephrotic syndrome hypercoagulability
Children who suffer from nephrotic syndrome lose proteins that contribute to the coagulation cascade. In addition, lipoprotein profiles are altered: there is a rise in the very low-density lipoproteins (LDL), contributing to accelerated atherosclerosis. Typically nephrotic patients have normal levels of high-density lipoproteins (HDL), unless there is profuse proteinuria.
Children with difficult-to-control nephrotic syndrome (typically steroid-resistant) may form accelerated plaques that rupture, causing focal MI, as early as school age.
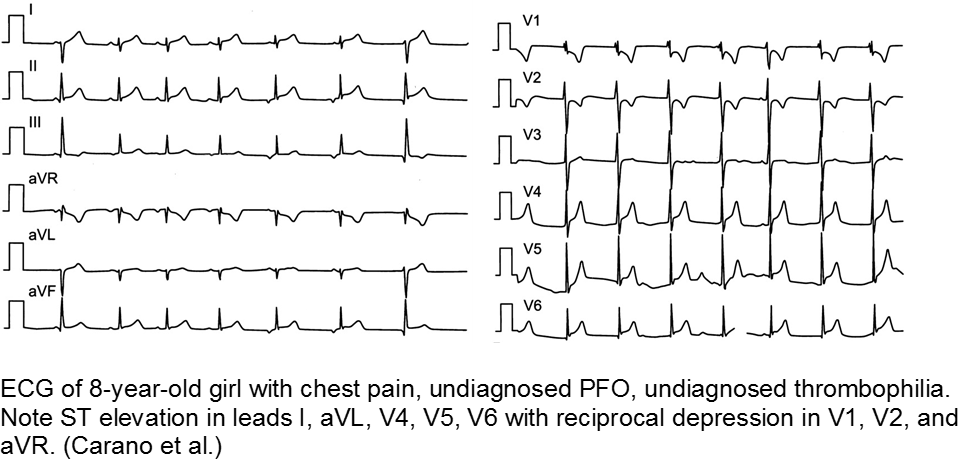
The previously well child now decompensated: undiagnosed thrombophilia
Asymptomatic patent foramen ovale (PFO) is the cause of some cases of cryptogenic vascular disease, such as stroke and MI. However, the presence of PFO alone does not connote higher risk. When paired with an inherited or acquired thrombogenic condition, the venous thrombus may travel from the right-sided circulation to the left, causing distal ischemia. Many of these cases are unknown until a complication arises.
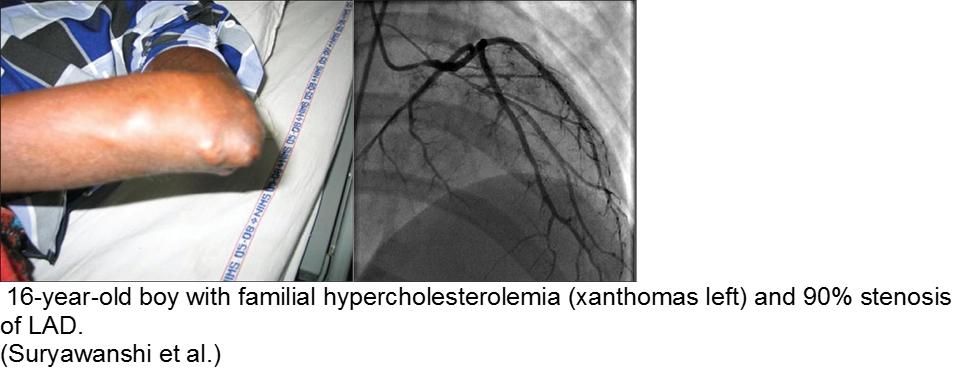
The chronically worried, now with a reason: hypercholesterolemia
A family history of adult-onset hypercholesterolemia is not necessarily a risk factor for early complications in children, provided the child does not have the same acquired risk factors as adults (e.g. obesity, sedentary lifestyle, smoking, etc). Parents may seek help in the ED for children with chest pain and no risk factors, but adult parents who have poor cholesterol profiles.
The exception is the child with familial hypercholesterolemia, who is at risk for accelerated atherosclerosis and MI.
Infectious
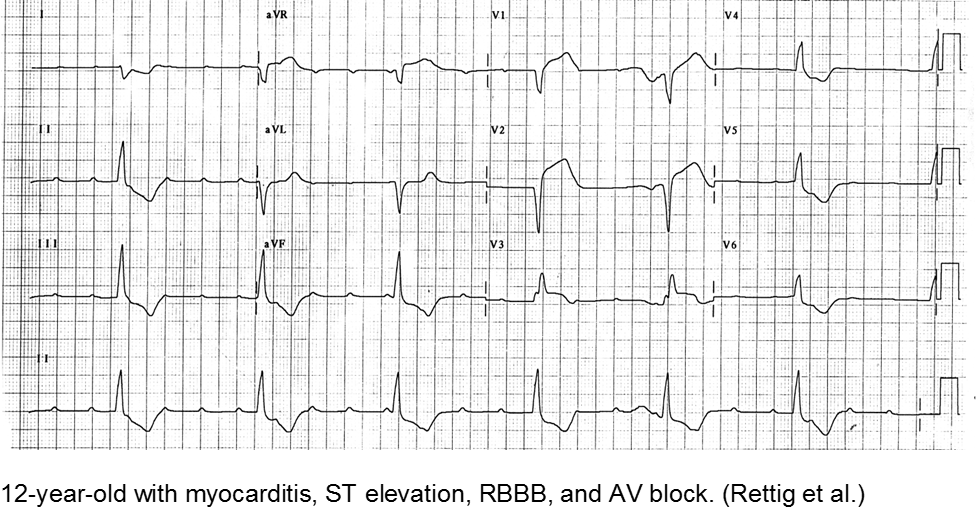
Myocarditis has varied etiologies, including infectious, medications (chemotherapy agents), immunologic (rheumatologic, transplant rejection), toxins (arsenic, carbon monoxide, heavy metals such as iron or copper), or physical stress (electrical injury, heat illness, radiation).
In children, the most common cause of myocarditis is infectious (viruses, protozoa, bacteria, fungal, parasites). Of these, viral causes are the most common (adenovirus, enterovirus, echovirus, rubella, HHV6).
The verbal child may complain of typical chest complaints, or may come in with flu-like illness and tachycardia or ill appearance out of proportion to presumed viral illness.
The most common presenting features in children with myocarditis are: shortness of breath, vomiting, poor feeding, hepatomegaly, respiratory distress, and fever.
The infant in shock after a ‘cold’: myocarditis
Beware of the poor feeding, tachycardic, ill appearing infant who “has a cold” because everyone else around him has a ‘cold’. That may very well be true, but any virus can be invasive with myocardial involvement. Infants are only able to increase their cardiac output through increasing their heart rate; they cannot respond to increased demands through ionotropy. Look for signs of acute heart failure, such as hepatomegaly, respiratory distress, and sacral edema.
The child with tachycardia out of proportion to complaint: myocarditis
The previously healthy child with “a bad flu” may simply be very symptomatic from influenza-like illness, or he may be developing myocarditis. Look for chest pain and tachycardia out of proportion to presumed illness, and constant chest pain, not just associated with cough.
The “pneumonia” with suspicious chest x-ray: myocarditis
Acute heart failure may mimic viral pneumonia. Look for disproportionate signs and symptoms.
Toxins
Younger children may get into others’ medications, be given dangerous home remedies, take drugs recreationally, have environmental exposures (heavy metals), suffer from a consequence of a comorbidity (iron or copper overload) or have adverse events from generally safe medications.
The hyperactive boy with a hyperactive precordium: methylphenidate
Attention deficit hyperactivity disorder (ADHD) is growing in rate of diagnosis and use of medications. As the only medical diagnosis based on self-reported criteria, many children are given stimulants regardless of actual neurologic disorder; with a higher proportion of children exposed to stimulants, adverse effects are seen more commonly.
Methylphenidate is related to amphetamine, and they both are dopaminergic drugs. Their mechanisms of action are different, however. Methylphenidate increases neuronal firing rate. Methamphetamine reduces neuronal firing rate; cardiovascular sequelae such as MI and CHF are more common in chronic methamphetamine use.
Although methylphenidate is typically well tolerated, risks include dysrhythmias such as ventricular tachycardia.
The child with seizure disorder and chest pain: anti-epileptics
Some anti-epileptic agents, such as carbamazepine, promote a poor lipid profile, leading to atherosclerosis and early MI. Case reports include school-aged children on carbamazepine who have foamy cells in the coronary arteries, aorta, and vasa vasorum on autopsy. It is unclear whether this is a strong association.
The spice trader: synthetic cannabinoids
Synthetic cannabinoids are notoriously difficult to regulate and study, as the manufacturers label them as “not for human consumption”. Once reports surface of abuse of a certain compound, the formula is altered slightly and repackaged, often in a colorful or mysterious way that is attractive to teenagers.
The misperceptions are: are a) synthetics are related to marijuana and therefore safe and b) marijuana is inherently “safe”. Both tend to steer unwitting teens to take these unknown entities. Some suffer MI as a result.
Exposure to tetrahydrocannabinol (THC) in high-potency marijuana has been linked to myocardial ischemia, ventricular tachycardia, and ventricular fibrillation. Marijuana can increase the heart rate from 20-100%, depending on the amount ingested.
K2 (“kush 2.0”) or Spice (Zohai, Genie, K3, Bliss, Nice, Black Mamba, fake weed, etc) is a mixture of plant leaves doused in synthetic chemicals, including cannabinoids and fertilizer (JWH-108), none of which are tested or safe for human consumption.
Synthetic cannabinoids have a higher affinity to cannabinoid receptors, conferring higher potency, and therefore worse adverse effects. They are thought to be 100 to 800 times more potent as marijuana.
Bath salts (Purple Wave, Zoom, Cloud Nine, etc) can be ingested, snorted, or injected. They typically include some form of cathinone, such as mephedrone, similar to the substance found in the naturally occurring khat plant. Hallucinations, palpitations, tachycardia, MI, and dysrhythmias have been reported from their use as a recreational drug.
Chest pain with marijuana, synthetic cannabinoid, or bath salt ingestion should be investigated and/or monitored.
Riding that train: high on cocaine
Cocaine is a well-known cause of acute MI in young people. In addition to the direct stimulant causes acutely, such as hypertension, tachycardia, and impaired judgement (coingestions, risky behavior), chronic cocaine use has long-term sequelae. Cocaine causes accelerated atherosclerosis. That, in conjunction with arterial vasospasm and platelet activation, is a recipe for acute MI in the young.
Cranky: methamphetamine
Methamphetamine is a highly addictive stimulant that is relatively inexpensive and widely available. Repeated use causes multiple psychiatric, personality, and neurologic changes. Risky behavior, violence, and motor vehicle accidents are all linked to this drug.
Like cocaine, methamphetamine may cause fatal dysrhythmias, acute MI from demand ischemia, and long-term sequelae such as congestive heart failure.
Summary
Acute MI is a challenging presentation in children:
- Easily missed: uncommon and atypical
- Varied etiology
- Respect vague symptoms with a non-reassuring H&P
- Try to detect it: CATH IT!
References
Congenital
AboulHosn JA et al. Fontan Operation and the Single Ventricle. Congenit Heart Dis. 2007; 2:2-11.
Aliku TO et al. A case of anomalous origin of the left coronary artery presenting with acute myocardial infarction and cardiovascular collapse. African Health Sci. 2014; 14(1): 23-227.
Andrews RE et al. Acute myocardial infarction as a cause of death in palliated hypoplastic left heart syndrome. Heart. 2004; 90:e17.
Canale LS et al. Surgical treatment of anomalous coronary artery arising from the pulmonary artery. Interactive Cardiovascaulr and Thoracic Surgery. 2009; 8:67-69.
Güvenç O et al. Correctable Cause of Dilated Cardiomyopathy in an Infant with Heart Failure: ALCAPA Syndrome. J Curr Pediatr. 2017; 15:47-50.
Hastings RS et al. Embolic Myocardial Infarction in a Patient with a Fontan Circulation. World Journal for Pediatric Congenital Heart Surgery. 2014; 5(4)L631-634.
Hoffman JIE et al. Electrocardiogram of Anomalous Left Coronary Artery From the Pulmonary Artery in Infants. Pediatr Cardiol. 2013; 34(3):489-491.
Kei et al. Rare Case of Myocardial Infarction in a 19-Year-Old Caused by a Paradoxical Coronary Artery Embolism. Perm J.2015; 19(2):e107-e109.
Liu Y, Miller BW. ALCAPA Presents in an Adult with Exercise Inlerance but Preserved Cardiac Function. Case Reports Cardiol. 2012; AID 471759.
Möhlenkamp S et al. Update on Myocardial Bridging.Circulation. 2002;106:2616-2622.
Murgan SJ et al. Acute myocardial infraction n the neonatal period. Cardiol Young. 2002; 12:411-413.
Sieweke JT et al. Myocardial infarction in grown up patients with congenital heart disease: an emergening high-risk combination. International Journal of Cardiology. 2016; 203:138-140.
Schwerzmann M et al. Anomalous Origin of the Left Coronary Artery From the Main Pulmonary Artery in Adults. Circulation. 2004; 110:e511-e513.
Tomkewicz-Pajak L et al. Arterial stiffness in adult patients after Fontan procedure. Cardiovasculr Ultrasound. 2014; 12:15.
Varghese MJ et al. The caveats in the diagnosis of anomalous origin of left coronary artery from pulmonary artery (ALCAPA). Images Paediatr Cardiol. 2010; 12(3): 3–8.
Autoimmune
Ayala et al. Acute Myocardial Infarction in a Child with Systemic Lupus Erythematosus and Antiphospholipid Syndrome. Turk J Rheumatol. 2009; 24:156-8.
Nakano H et al. Clinical characteristics of myocardial infarction following Kawasaki disease: Report of 11 cases. J Pediatr. 1986; 108(2):198-203.
Pongratz G et al. Myocardial infarction in an adult resulting from coronary aneurysms previously documented in childhood after an acute episode of Kawasaki’s disease. European Heart J. 1994. 15:1002-1004.
Newburger JW et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease. A Statement for Health Professionals From the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747-2771.
Son MB et al. Kawaski Disease. Pediatr Rev. 2013; 34(4).
Yuan S. Cardiac surgical procedures for the coronary sequelae of Kawasaki disease. Libyan J Med. 2012; 7:19796.
Trauma
Abdolrahim SA et al. Acute Myocardial Infarction Following Blunt Chest Trauma and Coronary Artery Dissection. J Clin Diagnost Res. 2016; 10(6):14-15.
Galiuto L et al. Post-traumatic myocardial infarction with hemorrhage and microvascular damage in a child with myocardial bridge: is coronary anatomy actor or bystander. Signa Vitae. 2013; 8(2):61-63.
Janella BL et al. Acute Myocardial Infarction related to Blunt Thoracic Trauma. Arq Bras Cardiol. 2006; 87:e168-e171.
Liu X et al. Acute myocardial infarction in a child with myocardial bridge World J Emerg Med. 2011; 2(1):70-72.
Long WA et al. Childhood Traumatic Infarction Causing Left Ventricular Aneurysm: Diagnosis by Two-Dimensional Echocardiography. JACC. 1985; 5(6):1478-83.
Smith S. Right Bundle Branch Block after Blunt Trauma: A Tragic Case. [Blog Post] July 22, 2012. Retrievable at: http://hqmeded-ecg.blogspot.com/2012/07/right-bundle-branch-block-after-blunt.html.
Hematologic
Carano N et al. Acute Myocardial Infarction in a Child: Possible Pathogenic Role of Patent Foramen Ovale Associated with Heritable Thrombophilia. Pediatr. 2004; 114(2):255-258.
Chacko P et al. Myocardial Infarction in Sickle Cell Disease. J Cardiovascl Transl Res. 2013; 6(5):752-761.
De Montalembert M et al. Myocardial ischaemia in children with sickle cell disease. Arch Dis Child. 2004; 89:359-362.
Gladwin MT et al. Cardiovascular Abnormalities in Sickle Cell Disease. JACC. 2012; 59(13):1123-1133.
Osula S et al. Acute myocardial infarction in young adults: causes and management. Postgrad Med J. 2002; 78:27-30.
Silva JMP et al. Premature acute myocardial infarction in a child with nephrotic syndrome. Pediatr Nephrol. 2002; 17:169-172.
Suryawanshi SP. Myocardial infarction in children: Two interesting cases. Ann Pediatr Cardiol. 2011 Jan-Jun; 4(1): 81–83.
Infectious
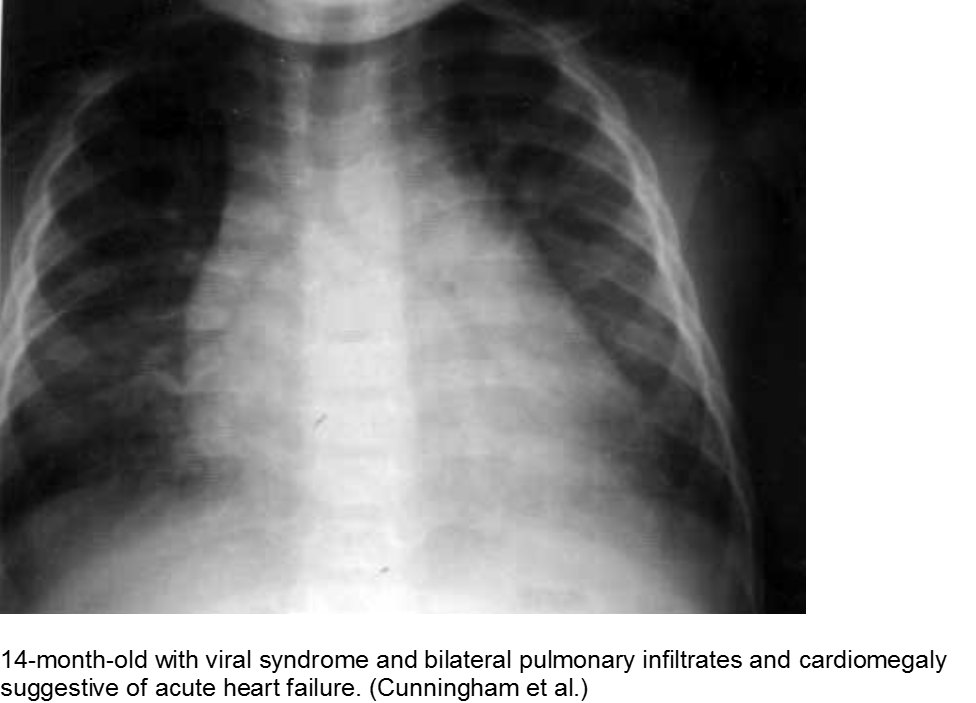
Cunningham R et al. Viral myocarditis Presenting with Seizure and Electrocardiographic Findings of Acute Myocardial Infarction in a 14-Month-Old Child. Ann Emerg Med. 2000; 35(6):618-622.
De Vettten L et al. Neonatal Myocardial Infarction or Myocarditis? Pediatr Cardiol. 2011; 32:492-497.
Durani Y et al. Pediatric myocarditis: presenting clinical characteristics. Am J Emerg Med. 2009; 27:942-947.
Erden I et al. Acute myocarditis mimicking acute myocardial infarction associated with pandemic 2009 (H1N1) influenza virus. Cardiol J. 2011; 552-555.
Hover MH et al. Acute Myocarditis Simulating Myocardial Infarction in a Child. Pediatr. 1191; 87(2):250-252.
Lachant D et al. Meningococcemia Presenting as a Myocardial Infarction. Case Reports in Critical Care. 2015; AID 953826.
Laissy JP et al. Differentating Myocardial Infarction from Myocarditis. Radiology. 2005; 237(1):75-82.
Miranda CH et al. Evaluation of Cardiac Involvement During Dengue Viral Infection. CID. 2013; 57:812-819.
Rettig JS et al. Myocarditis in Children Requiring Critical Care Transport. In: "Diagnosis and Treatment of Myocarditis", Milei J, Ambrosio G (Eds). DOI: 10.5772/56177.
Toxins
De Chadarévian JP et al. Epilepsy, Atherosclerosis, Myocardial Infarction, and Carbamazepine. J Child Neurol. 2003; 18(2):150-151.
McIlroy G et al. Acute myocardial infarction, associated with the use of a synthetic adamantly-canabinoid: a case report. BMC Pharmacology and Toxicology. 2016; 17:2.
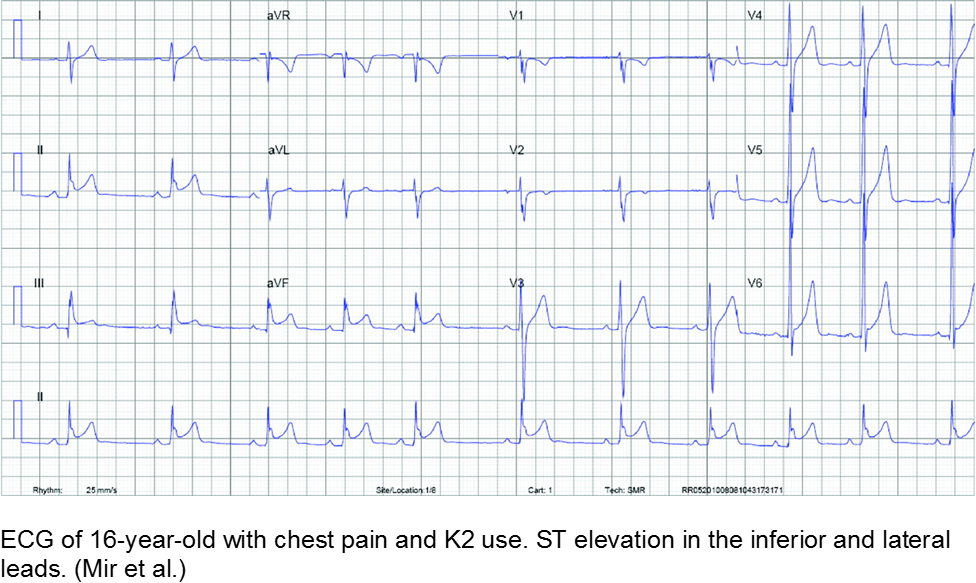
Mir A et al. Myocardial Infarction Associated with Use of the Synthetic Cannabinoid K2. Pediatr. 2011; 128(6):1-6
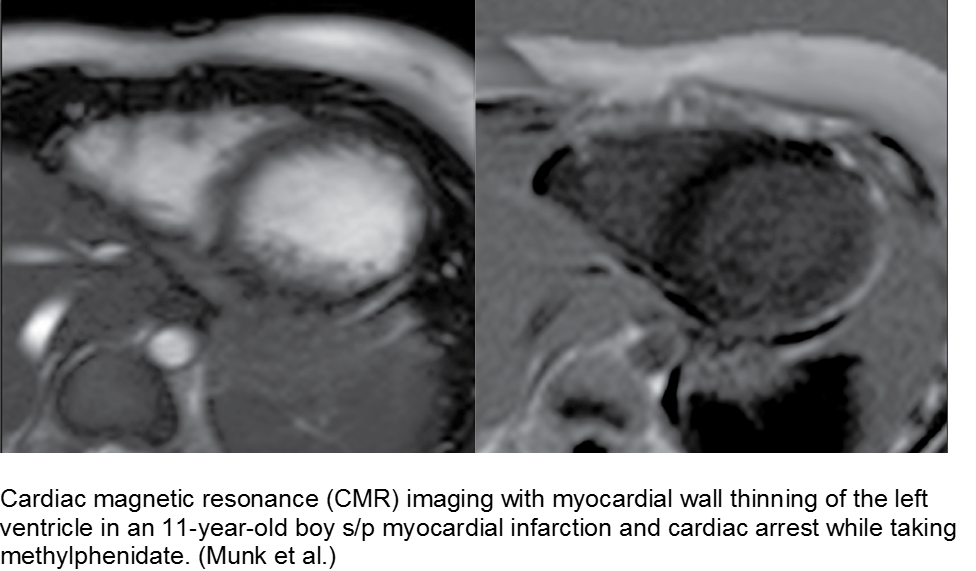
Munk K et al. Cardiac Arrest following a Myocardial Infarction in a Child Treated with Methylphenidate. Case Reports Pediatr. 2015; AID 905097.
Rezkalla SH et al. Cocaine-Induced Acte Mycardial Infarction. Clin Med Res. 2007; 5(3):172-176.
Schelleman H et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012 Feb;169(2):178-85.
Sheridan J et al. Injury associated with methamphetamine use: a review of the literature. Harm Reduction Journal, 2006; 3(14):1-18.
Stiefel G et al. Cardiovascular effects of methylphenidate, amphetamines and atomoxetine in the treatment of attention-deficit hyperactivity disorder. Drug Saf. 2010 Oct 1;33(10):821-42.
This post and podcast are dedicated to Edwin Leap, MD for his sanity and humanity in the practice of Emergency Medicine. Thank you, Dr Leap for all that you do.
It's a busy shift. Today no one seems to have a chief complaint.
Someone sends a troponin on a child. Good, bad, or ugly, how are you going to interpret the result?
And while we’re at it – what labs do I need to be careful with in children – sometimes the normal ranges of common labs can have our heads spinning!
Read on to go from bread-and-butter pediatric blood work to answer the question – what’s up with troponin, lactate, d-dimer, and BNP in kids?
A fundamental tenet of emergency medicine:
We balance our obligation to detect a dangerous condition with our suspicion of the disease in given patient.
Someone with a cough and fever may simply have a viral illness, or he may have pneumonia. Our obligation is to evaluate for the pneumonia. It’s ok if we “miss” the diagnosis of a cold. It could be bad if we don’t recognize the pneumonia.
How do we decide? Another fundamental concept:
The threshold.
Depending on the disease and the particular patient, we have a threshold for testing, and the threshold for treating. Every presentation – and every patient for that matter – has a complicated interplay between what we are expected to diagnose, how much we suspect that particular serious diagnosis, and where testing and treating come into play.
What's wrong with "throwing on some labs"?
Easy to do right? They are but a click away…
Often a good history and physical exam will help you to calibrate your investigational thresholds. This is especially true in children – the majority of pediatric ambulatory visits do not require blood work to make a decision about acute care. If your patient is ill, then by all means; otherwise, consider digging a bit deeper into the history, get collateral information, and make good use of your general observation skills.
First, a brief word about basic labs.
The punchline is, use a pediatric reference.
If you don’t have a trusted online reference available during your shift, make sure you have something like a Harriett Lane Handbook accessible to you. Don’t rely on your hospital’s lab slip or electronic medical record to save you, unless you are sure that they use age-specific pediatric reference ranges to flag abnormal values. Believe it or not, in this 21st century of ours, some shops still use adult reference ranges when reporting laboratory values on children.
Notable differences in basic chemistries
Potassium: tends to run a bit higher in infants, because for the first year of life, your kidneys are inefficient in excreting potassium.
BUN and creatinine: lower in children due to less muscle mass, and therefore less turnover (and usually lack of other chronic disease)
Glucose: tends to run lower, as children are hypermetabolic and need regular feeding (!)
Alkaline phosphatase: is always high in normal, growing children, due to bone turn over (also fond in liver, placenta, kidneys)
Ammonia: high in infancy, due to immature liver, trends down to normal levels by toddlerhood
ESR and CRP: low in healthy children, as chronic inflamation from comorbidities is not present; both increase steadily with age
Thyroid function tests: all are markedly high in childhood, not as a sign of disease, but a marker of their increased metabolic activity
Big Labs
Troponin
Reliably elevated in myocarditis, and may help to distinguish this from pericarditis (in addition to echocardiography)
Other causes of elevated troponin in children include: strenuous activity, status epilepticus, toxins, sepsis, myocardial infarction (in children with congenital anomalies). Less common causes of troponemia are: Kawasaki disease, pediatric stroke, or neuromuscular disease.
Don't go looking, if you won't do anything with the test.
Brain natriuretic peptide (BNP)
In adults, we typically think of a BNP < 100 pg/mL as not consistent with symptoms caused by volume overload.
Luckily, we have data in children with congenital heart disease as well. Although each company's assay reports slightly different cut-offs, in general healthy pediatric values match healthy adult values.
One exception is in the first week of life, when it is high even in healthy newborns, due to the recent transition from fetal to newborn circulation.
Use of BNP in children has been studied in both clinic and ED settings. Cohen et al. in Pediatrics used BNP to differentiate acute heart failure from respiratory disease in infants admitted for respiratory distress. They compared infants with known CHF, lung disease, and matched them with controls.
Later, Maher et al. used BNP in the emergency department to differentiate heart failure from respiratory causes in infants and children with heart failure and those with no past medical history.
The bottom line is:
BNP reliably distinguishes cardiac from respiratory causes of shortness of breath in children with a known diagnosis of heart failure.
D-dimer
To cut to the chase: d-dimer for use as a rule-out for pulmonary embolism has not been studied in children.
The only data we have in using d-dimer in children is to prognosticate in established cases. It is only helpful to track therapy for children who have chronic clots.
This is where our adult approach can get us into trouble. Basically, think of the d-dimer in children like it doesn’t even exist. It’s not helpful in our setting for our indications. An adult may have an idiopathic PE – in fact, up to a third of adults with PE have no known risk factor, which makes decision tools and risk stratification important in this population.
Children with PE almost always have a reason for it.
There is at least one identifiable risk factor in up to 98% of children with pulmonary embolism. The majority have at least two risk factors.
If you’re suspecting deep venous thrombosis, perform ultrasonography, and skip the d-dimer.
If you’re worried about PE, go directly to imaging. In stable patients, you may elect to use MR angiography or VQ scan, but most of us will go right to CT angiography. Radiation is always a concern, but if you need to know, get the test.
This is yet another reminder that your threshold is going to be different in children when you think about PE – they should have a reason for it. After you have excluded other causes of their symptoms, if they have risk factors, and you are still concerned, then do the test you feel you need to keep this child safe.
You are the test.
Risk factors only inform you, and you’ll have to just pull the trigger on testing in the symptomatic child with risk factors.
Lactate
A sick child with sepsis syndrome?
The short answer – yes.
In the adult literature, we know that a lactate level above 4 mmol/L in patients with severe sepsis was associated with the need for critical care. This has been studied in children as well, and an elevated lactate in children – typically above 4 – was a predictor of prolonged ICU course and mortality in septic patients.
The acute recognition and treatment of sepsis is first and foremost, clinical.
And it’s all about perfusion and providing oxygen to the tissues. Lactate and other laboratory testing is not a substitute for clinical assessment – it should be used as an extension of your assessment. There are two main reasons for an elevated lactate: the stress state and the shock state.
The stress state is due to hypermetabolism and an increase in glycolysis, as an example, in early sepsis. The shock state is due to tissue hypoxia, seen in septic shock. The confusion and frustration with lactate is that we often test the wrong people for it.
We could use it to track treatment, and see if we can clear the lactate; decreased lactate levels are associated with a better outcome in adults. Serial clinical assessments are even more useful to gauge your success with treatment.
We should use lactate to detect occult shock. Children compensate so well for shock, that subtle tissue hypoxia may not be detected until later. It may inform your decision for level of care, intensive care versus some other lower level.
Have you every been in this situation:
"Why, oh why, did we send a lactate?"
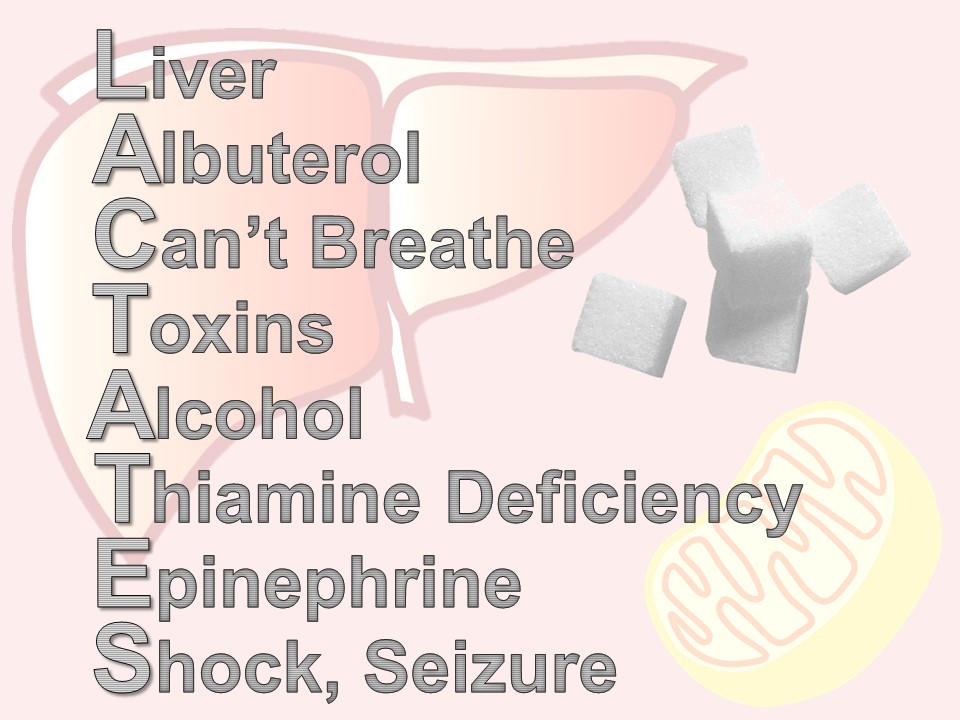
There are times when a lactate is ordered – maybe by protocol or maybe accidentally – or maybe in retrospect, the patient didn’t need it. Here is a quick mnemonic to remember the reasons for an elevated lactate: LACTATES
L – liver – any liver disease affects how lactate is metabolized by the Cori cycle
A – albuterol (or for our international friends, salbutamol), beta-agonists like albuterol, increase lactate production via cyclic amp
C – “can’t breathe” – respiratory distress and increased work of breathing shifts the ratio of aerobic and anerobic repiration
T – toxins – all kinds of wonder drugs and recreational drugs do it – look up your patient’s list if you’re suspicious
A – alcohol, not an infrequent offender
T – thiamine deficiency – think of this in your cachectic or malnourished patients
E – epinephrine – a by-product of the cori cycle, how lactate is metabolized. Difficult to interpret lactates when a patient is on an epinephrine drip.
S – seizure or shock – most commonly septic, but can be any type: cardiogenic, bstructive, hypovolemic, distributive.
Bottom line: high serum lactate levels have been associated with morbidity and mortality in children with sepsis and trauma, the two best-studied populations.
A summary of how labs can help you – or hurt you – in pediatric emergency medicine:
- Have a good reference for normal values and always be skeptical of how your lab reports them.
- Troponin testing is great for the child with suspected cardiogenic shock, myocarditis, or in unwell children with congenital heart disease.
- BNP in children can be used just like you do in adults – to get a sense of whether the presenting symptoms are consistent with heart failure.
- D-dimer is mostly a waste of time in the PED.
- Lactate can be useful in the right patient – use it to risk-stratify the major trauma patient or the patient with sepsis that may be suffering from occult shock.
- And lastly, make sure that you are mindful of your threshold for testing, and our threshold for treatment. If will vary by disease and by the patient at hand.
References
Troponin
Gupta SK, Naheed Z. Chest Pain in Two Athletic Male Adolescents Mimicking Myocardial Infarction. Pediatr Emer Care. 2014;30: 493-495.
Kelley WE, Januzzi JL, Christenson RH. Increases of Cardiac Troponin in Conditions other than Acute Coronary Syndrome and Heart Failure. Clinical Chemistry. 2009; (55) 12:2098–2112.
Kobayashi D, Aggarwal S, Kheiwa A, Shah N. Myopericarditis in Children: Elevated Troponin I Level Does Not Predict Outcome. Pediatr Cardiol. 2012; 33:1040–1045.
Koerbin G, Potter JM, Abhayaratna WP et al. The distribution of cardiac troponin I in a population of healthy children: Lessons for adults. Clinica Chimica Acta. 2016; 417: 54–56.
Liesemer K, Casper TC, Korgenski K, Menon SC. Use and Misuse of Serum Troponin Assays in Pediatric Practice. Am J Cardiol. 2012;110:284 –289.
Newby KL et al. for the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF 2012 Expert Consensus Document on Practical Clinical Considerations in the Interpretation of Troponin Elevations. J Am Coll Cardiol. 2012; 60(23): 2427-2463.
Schwartz MC, Wellen S, Rome JJ et al. Chest pain with elevated troponin assay in adolescents. Cardiology in the Young; 2013. 23: 353–360.
BNP
Auerbach SR, Richmond ME, Lamour JM. BNP Levels Predict Outcome in Pediatric Heart Failure Patients Post Hoc Analysis of the Pediatric Carvedilol Trial. Circ Heart Fail. 2010;3:606-611.
Cohen S, Springer C, Avital A et al. Amino-Terminal Pro-Brain-Type Natriuretic Peptide: Heart or Lung Disease in Pediatric Respiratory Distress? Pediatrics. 2005;115:1347–1350.
Fried I, Bar-Oz B, Algur N et al. Comparison of N-terminal Pro-B-Type Natriuretic Peptide Levels in Critically Ill Children With Sepsis Versus Acute Left Ventricular Dysfunction. Pediatrics. 2006; 118(4): 1165-1168.
Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–878.
Maher KO, Reed H, Cuadrado A et al. , B-Type Natriuretic Peptide in the Emergency Diagnosis of Critical Heart Disease in Children. Pediatrics. 2008;121:e1484–e1488.
Mir TS, Marohn S, Laeer S, Eistelt M. Plasma Concentrations of N-Terminal Pro-Brain Natriuretic Peptide in Control Children From the Neonatal to Adolescent Period and in Children With Congestive Heart Failure. Pediatrics. 2002;110(6)1:6.
Mir TS, Laux R, Hellwege HH et al. Plasma Concentrations of Aminoterminal Pro Atrial Natriuretic Peptide and Aminoterminal Pro Brain Natriuretic Peptide in Healthy Neonates: Marked and Rapid Increase After Birth. Pediatrics. 2003;112:896–899.
D-Dimer
Goldenberg NA, Knapp-Clevenger RA, Manco-Johnson MJ. Elevated Plasma Factor VIII and d-Dimer Levels as Predictors of Poor Outcomes of Thrombosis in Children for the Mountain States Regional Thrombophilia Group. Pediatrics. 2003;112:896–899.
Manco-Johnson MJ. How I treat venous thrombosis in children. Blood. 2006; 107(1)21-31.
Naqvi M, Miller P, Feldman L, Shore BJ. Pediatric orthopaedic lower extremity trauma and venous thromboembolism. J Child Orthop. 015;9:381–384.
Parasuraman S, Goldhaber SZ. Venous Thromboembolism in Children. Circulation. 2006;113:e12-e16.
Strouse JJ, Tamma P, Kickler TS et al. D-Dimer for the Diagnosis of Venous Thromboembolism in Children. N Engl J Med. 2004;351:1081-8.
Lactate
Andersen LW, Mackenhauer J, Roberts JC et al. Etiology and therapeutic approach to elevated lactate. Mayo Clin Proc. 2013; 88(10): 1127–1140.
Bai et al. Effectiveness of predicting in-hospital mortality in critically ill children by assessing blood lactate levels at admission. BMC Pediatrics. 2014; 14:83.
Scott HF, Donoghue AJ, Gaieski DF et al. The Utility of Early Lactate Testing in Undifferentiated Pediatric Systemic Inflammatory Response Syndrome. Acad Emerg Med. 2012; 19:1276–1280.
Shah A, Guyette F, Suffoletto B et al. Diagnostic Accuracy of a Single Point-of-Care Prehospital Serum Lactate for Predicting Outcomes in Pediatric Trauma Patients. Pediatr Emer Care. 2013; 29:715-719.
Topjian AA, Clark AE, Casper TC et al. for the Pediatric Emergency Care Applied Research Network. Early Lactate Elevations Following Resuscitation From Pediatric Cardiac Arrest Are Associated With Increased Mortality. Pediatr Crit Care Med. 2013; 14(8): e380–e387.
This post and podcast are dedicated to Daniel Cabrera, MD for his vision and his leadership in thinking 'outside the box'.
Troponin | BNP | D-Dimer | Lactate
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
In the young child, vomiting is the great imitator:
Gastrointestinal, Neurologic, Metabolic, Respiratory, Renal, Infectious, Endocrine, Toxin-related, even Behavioral.
To help us organize, below is a review of can't-miss diagnoses by age.
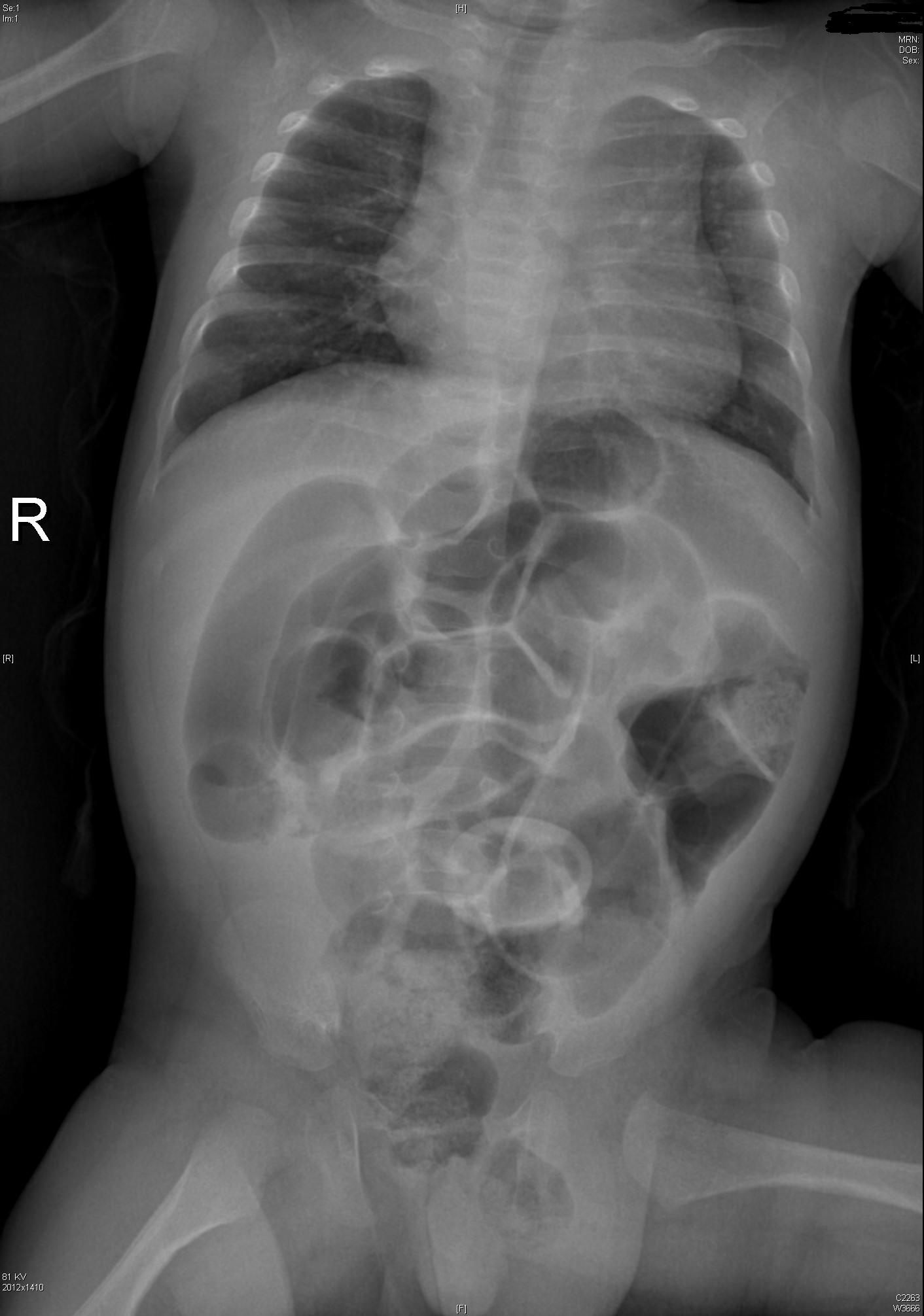
The Neonate: Malrotation with Volvulus
In children with malrotation, 50% present within the first month of life, with the majority occurring in the first week after birth. 90% of children with malrotation with volvulus will present by one year of age. This is a pre-verbal child’s disease – which makes it even more of a challenge to recognize quickly.
The sequence of events usually is fussiness, irritability, and forceful vomiting. The vomit quickly turns bilious.
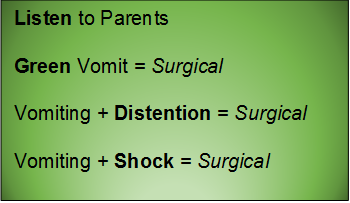
Green vomit is a surgical emergency.
Babies may also present unwell, with bloating and abdominal tenderness to palpation. Be aware that later stages of malrotation may present as shock – they present in hypovolemic shock due to third-spacing from necrotic bowel and/or septic shock from translocation or perforation. In the undifferentiated sick neonate, always consider a surgical emergency such as malrotation with volvulus.
In the stable patient, get an upper GI contrast study.
Rapid-fire word association for other vomiting emergencies in a neonate:
- Fever, irritability and vomiting? Think meningitis, UTI, or sepsis.
- Premature, unwell, and vomiting? Think necrotizing enterocolitis. Remember, 10% of cases of NEC can be full-term. Look for pneumatosis intestinalis.
- Systemically ill, afebrile, and vomiting for no other reason? Think inborn error of metabolism. Screen with a glucose, ammonia, lactate, and urine ketones.
- Others include congenital intestinal atresia or webs, meconium ileus, or severe GERD
The Infant: Non-Accidental Trauma
All that vomits is not necessarily from the gut.
Abusive head injury is the most common cause of death from child abuse. Infants especially present with non-specific complaints like fussiness or vomiting. Up to 30% of infants with abusive head injury may be misdiagnosed on initial presentation.
Louwers et al. in Child Abuse and Neglect developed and validated a six-question screening tool for use the in ED. The power of this tool was that it was validated for any chief complaint – it is not an injury evaluation checklist – it is a screen for potential abuse in the undifferentiated child:
- Is the history consistent?
- Was seeking medical help unnecessarily delayed?
- Does the onset of injury fit with the developmental level of the child?
- Is the behavior of the child and his interaction with his care-givers appropriate?
- Do the findings of the head-to-toe examination match the history?
- Are there any other red flags or signals that make you doubt the safety of the child or other family members?
On multivariable analysis, if at least one of the questions was positive, there was an OR of 189 for abuse (CI 97 – 300). In other words, if any of those six questions are problematic, get your child protective team involved.
Other important diagnoses in the infant: intussusception and pyloric stenosis (rapid review in audio).
The Toddler: Diabetic Ketoacidosis (DKA)
The important diagnosis not to miss in the vomiting toddler or early school age child is the initial presentation of diabetic ketoacidosis. Children under 5 (especially those under 2) and those from lower socioeconomic groups have a higher risk of DKA as their initial presentation of diabetes.
This is true for any child that isn’t quite acting right – check a finger stick blood sugar as a screen.
The International Society for Pediatric and Adolescent Diabetes (ISPAD) criteria for DKA:
- Hyperglycemia, with a blood glucose of >200 mg/dL (11 mmol/L)
AND - Evidence of metabolic acidosis, with a venous pH of less than 7.3 or a bicarbonate level of < 15 mEq/L
AND - Ketosis, found either in the urine or if directly checked in the blood.
If you have access to checking a serum beta-hydroxybutryrate – the unsung ketone – it can help in diagnosis in unclear cases.
Cerebral Edema Criteria:
- Minor criteria: headache, vomiting, irritability or lethargy; hypertension in the face of hypovolemia.
- Major criteria: change in mental status, including agitation or delirium; incontinence (especially if inappropriate for the child’s age); sluggish pupils and cranial nerve palsies; relative bradycardia (Cushing’s triad).
Cerebral Edema Action Items:
- Immediately give mannitol, 1 g/kg over 15-20 minutes. May repeat it in 2 hours if needed. Hypertonic saline (3% NaCl) is second-line therapy.
- Put the head of the bed up 30 degrees.
- Alert your colleagues and counsel your parents. Make sure everyone knows what to watch out for.
As you can see, vomiting in the young child can be really anything! Keep your differential broad, and think by age and by system.
Differential Diagnosis of Vomiting in Children
The general approach to the child with chiefly vomiting starts with the decision: sick or not sick. If ill appearing, establish rapid IV access, or if needed IO. Rapid blood sugar and if available a point of care pH and electrolytes. Be the detective in your history and doggedly go after any red flags as you go methodically by organ system.
- Do a careful physical exam. The general assessment is always helpful – is the child irritable, listless, agitated?
- What is his work of breathing? Effortless tachypnea may be a sign of acidosis or sepsis.
- Is the abdomen soft or is it tender or distended. Always look in the diaper area – is there a hernia, is there a high-riding, tender, discolored scrotum without cremasteric reflex? Ovarian torsion has been reported in infants as young as 7 months.
- Any skin signs? Look for petechiae, urticaria, purpura.
In other words, use your best judgement, have the dangerous differentials in the back of your mind, and pull the trigger when red flags mount up. Otherwise, a good history and a good exam will get you where you need to be.
Take home points for the young child with vomiting:
- Neonates are allowed to regurgitate (effortless reflux of stomach contents -- the happy spitter-upper). They are not allowed to vomit (forceful, unpleasant contraction of abdominal muscles). Consider surgical causes of forceful vomiting, especially if the child does not look anything other than well.
- Bilious is bad – green vomit is always a surgical emergency – do not pass go – get the surgeons involved early
- Not all vomiting is GI related – if it is not obviously benign, think methodically by organ system and adjust your targeted history and physical to pick up any leads.
- Match the tempo of your treatment to the tempo of the disease.
References
Applegate KE, Anderson JM, Klatte EC. Intestinal malrotation in children: a problem-solving approach to the upper gastrointestinal series. Radiographics. 2006; 26(5):1485-500.
Glaser NS, Wootton-Gorges SL, Buonocore MH et al. Frequency of sub-clinical cerebral edema in children with diabetic ketoacidosis. Pediatr Diabetes. 2006 Apr;7(2):75-80.
Louwers ECFM, Korfage IJ, Affourtit MJ et al. Accuracy of a screening instrument to identify potential child abuse in emergency departments. Child Abuse & Neglect. 2014; (38): 1275–1281.
Lee HC, Pickard SS, Sridhar S et al. Intestinal Malrotation and Catastrophic Volvulus in Infancy. J Emerg Med. 2012; 43(1): e49–e51.
Marcin JP, Glaser N, Barnett P et al. Factors associated with adverse outcomes in children with diabetic ketoacidosis-related cerebral edema. J Pediatr. 2002; 141(6):793-7.
Parashette KR, Croffie J. Intestinal Malrotation in Children: A Problem-solving Approach to the Upper Gastrointestinal. Pediatrics in Review. 2013; (34)7: 307-321.
Wolfsdorf JI, Allgrove J, Craig ME et al. ISPAD Clinical Practice Consensus Guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014 Sep;15 Suppl 20:154-79.
This post and podcast are dedicated to Damian Roland, BMedSci (Hons), MB BS, MRCPCH, for his fervor in the care of children and his dedication to quality medical education.
Nausea and Vomiting | Non-Accidental Trauma | DKA
Powered by #FOAMed -- Tim Horeczko, MD, MSCR, FACEP, FAAP
Intranasal medications, if understood and employed properly, are a great choice to avoid and IV or as a bridge until IV access is obtained. Learn the strengths and limits of intranasal fentanyl, midazolam, ketamine, and dexmedetomidine.
Pain Management in Children
Traditionally, “brutaine”.
Goal: the “ouchless ED”.
Two main barriers in pain treatment in children:
1. We consistently under-recognize children’s pain. We may not detect the typical behaviors that children exhibit when they are in pain, especially in the pre-verbal child: crankiness or fussiness; changes in appetite or sleep; decreased activity; or physiologic findings such as dull eyes, flushed skin, rapid breathing, or sweating.
2. We under-treat pain in children. This is mostly from an old culture of misunderstanding or fear of overdose.
Four Components to Successful Pain Management and Intranasal Medication Administration
Right drug, right dose, right patient, right timing
Right Drug – Not every medication is easily amenable to intranasal administration. We can use intranasal drugs for analgesia, for anxiolysis, for seizures – but not all drugs used for those purposes will perform well – or at all – via the IN route.
Right Dose – Dosing with IN meds will vary considerably from the IV route. Rule of thumb: the IN dose is 2-3 times the IV dose.
Right Patient – Is this patient and family appropriate for “just taking the edge off”? What is the level of anxiety in the room? How is the child relating to the parent, usually it’s the mother there. What else is going on in that clinical snapshot as you walk in?
Right Timing – Mostly IV and IN onset times are very similar. Notable exception: intranasal midazolam may take 10-15 minutes to take effect – something to keep in mind when you plan your procedure.
Intranasal Medications bypass first-pass metabolism, and a portion of the drug is delivered into the CSF immediately via the nose-brain pathway.
Ideal Volume for Intranasal Medication: 0.25 to 0.3 mL per naris
Absolute maximum: 1 mL per naris (but expect some run-off)
Preload the device with 0.1 mL solution for dead space
Administer intranasal medications in the sniffing position. Lie the patient flat with occiput posterior, put patient in the sniffing position, seat the mucosal atomizing device cushion in the naris, aim toward the pinna of the ear, and shoot fast – you have to push the drug as fast as you can to atomize the solution.
Intranasal Fentanyl
Safe, effective at 2 mcg/kg. Most commonly stocked concentration of fentanyl is 50 mcg/mL. A 40-kg-child will reach the maximum volume possible for administration (40 kg x 2 mcg/kg = 80 mcg; at 50 mcg/mL – that makes 1.6 mL – if we divide the dose, it’s not ideal, but is still under our maximum of under 1 mL per naris.) You graduate from intranasal fentanyl in elementary school.
Sufentanil for adults (half the volume of fentanyl) – 0.5 mcg/kg, which can be repeated as needed.
Intranasal Midazolam
Intranasal Midazolam or versed for anxiolysis is dosed at 0.3 mg/kg (up to 0.5 mg/kg for procedural sedation)
Here, another practicality weighs in. The IV preparation for midazolam is 5 mg/5 mL – this a very dilute solution. You need to use the 5 mg/mL concentration to have any success with intransal midazolam because of the volume needed for the right effect.
A 20-kg-child will near the maximum volume for intranasal midazolam (0.3 mg/kg is 6 mg, at 5 mg/ml, 1.2 mL, or 036 mL per naris). Kindergarten graduation is when to drop the intranasal midazolam.
Intranasal Ketamine
The IV dose for ketamine for pain control is 0.15 to 0.3 mg/kg, usually as an infusion over an hour. The intranasal dose of ketamine for pain control is 1 mg/kg.
Low-dose ketamine may be used for pain control as an adjunct and opioid-sparing agent.
Intranasal Dexmedetomidine
Dexmedetomidine is an alpha-2 receptor agonist, a smarter clonidine. Clonidine is also an alpha-2 agonist, and it can cause a marked decrease in blood pressure with some mild sedation. Dexmedetomidine targets receptors in the CNS and spinal cord, and so it provides deep sedation, with very minimal blood pressure effects. It induces a sleep-like state. In fact, EEGs done under dex show the same pattern as seen in stage II sleep. Dex is safe, if titrated, and does not depress airway reflexes or respiration. Dose is 2.5 mcg/kg IN, and can add another 1 mcg/kg if needed. The downside is that it can last 30 minutes or more, but it may be a good choice for an abdominal ultrasound or CT head in unruly toddlers.
Before You Go: The “Semmelweiss reflex”.
Selected References
Weisman SJ, Bersnstein B, Schechter NL. Consequences of Inadequate Analgesia During Painful Procedures in Children. Biol Neonate. 2000 Feb;77(2):69-82.
Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Expert Opin Drug Deliv. 2008 Oct;5(10):1159-68. doi: 10.1517/17425247.5.10.1159 .
Wu H, Hu K, Jiang X. From nose to brain: understanding transport capacity and transport rate of drugs. J Opioid Manag. 2012 Jul-Aug;8(4):237-41. doi: 10.5055/jom.2012.0121.
Stephen R, Lingenfelter E, Broadwater-Hollifield C, Madsen T. Intranasal sufentanil provides adequate analgesia for emergency department patients with extremity injuries.
Do you have a plan for your little patient when he just won’t stop seizing? What do you do when your typical treatment is not enough? Get up-to-date in the understanding and management of pediatric status epilepticus.
Definition of status epilepticus:
Continuous seizure activity of 5 minutes or greater
– OR –
Recurrent activity without recovery between intervals. (This definition includes clinically apparent seizures as well as those seen only on EEG.)
During a seizure, GABA receptors in the neuron’s membrane are internalized and destroyed. Seizure activity itself starts this self-defeating process – this is the first reason we need to act as quickly as possible and take advantage of the GABA receptors that are still recruitable.
Excitatory receptors – the NMDA receptors – are acutely upregulated and mobilize to the neuron’s surface. This is the second reason to act quickly and avoid this kindling effect.
In other words – time is brain.
Or… is it something else as well?
Pediatric status epilepticus is analogous to the multi-organ dysfunction syndrome in severe sepsis. Status epilepticus affects almost every organ system.
Cardiac – dysrhythmias, high output failure, and autonomic dysregulation resulting in hypotension or hypertension.
Respiratory – apnea and hypoxia, ARDS, and potentially aspiration pneumonia.
Renal – rhabdomyolysis, myoglobinuria, and acute renal failure.
Metabolic – lactic acidosis, hypercapnia, hyperglycemia, sometimes hypoglycemia, hyperkalemia, and leukocytosis.
Autonomic – hyperpyrexia and breakdown of cerebral circulation.
DeLorenzo et al.: Mortality correlated with time seizing. Once the seizure has met the 30 min mark, Delorenzo reported a jump from 4.4% mortality to 22%! If the seizure lasts greater than 2 hours, 45%. Time spent seizing is a vicious cycle: it’s harder to break the longer it goes on, and the longer it goes on, the higher the mortality.
Think about treatment of pediatric status epilepticus in terms of time: prehospital care, status epilepticus (greater than 5 min), initial refractory status epilepticus (greater than 10 min), later refractory status (at 20 min), and coma induction (at 25 minutes).
Case 1: Hyponatremic Status Epilepticus
Give 3 mL/kg of 3% saline over 30 min.
Stop the infusion as soon as the seizure stops.
Case 2: INH toxicity
Empiric treatment -- you are the test. If we know the amount of ingestion in adults or children, we give a gram-for-gram replacement, up to 5 grams.
If a child under 2 years of age arrives to you in stats epilepticus, give 100 mg of IV pyridoxime for potentially undiagnosed congenital deficiency.
Case 3: Headache and Arteriovenous Malformation
Unlike in adults, stroke in children is divided evenly between hemorrhagic and ischemic etiologies.
The differential is vast: cardiac, hematologic, infectious, vascaulr, syndromic, metabolic, oncologic, traumatic, toxic.
Treatment: stabilization, embolization by interventional radiology, elective extirpation when more stable. Other options for stable patients include an endovascular flow-directed microcatheter using cyanoacrylate. Radiosurgery is an options for others.
Non-convulsive Status Epilepticus
Risk factors include age < 18, especially age < 1, no prior history of seizures, and traumatic brain injury. This would prompt you to ask for continuous EEG monitoring for non-convulsive status epilepticus, especially when there is a change in mental status for no other reason. Also, a prolonged post-ictal state or prolonged altered mental status. Other considerations are those who had a seizure and cardiac arrest - ROSC without RONF, those with traumatic brain injury, and those needing ECMO – all within the context of seizures.
SUMMARY POINTS
The longer the seizure lasts, the harder it is to break – act quickly
Have a plan for normal escalation of care, and Search for an underlying cause
Recognize when the routine treatment is not enough.
Before You Go
“Healing is a matter of time, but it is sometimes also a matter of opportunity.”
“Extreme remedies are very appropriate for extreme diseases.”
– Hippocrates of Kos
Selected References
Abend NS et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011; 76(12):1071-7
Baren J. Pediatric Seizures and Strokes: Beyond Benzos and Brain Scans. ACEP Scientific Assembly. October 8th, 2009. Boston, MA.
Brophy et al. Guidelines for the Evaluation and Management of Status Epilepticus. Neurocrit Care. 2012; DOI 10.1007/s12028-012-9695-z
Capovilla G et al. Treatment of convulsive status epilepticus in childhood: Recommendations of the Italian League Against Epilepsy. Epilepsia. 2013; 54 Suppl 7:23-34
Chin RFM et al., for the NLSTEPSS Collaborative Group. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006; 368: 222–29.
Chen JW, Chamberlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006; 5:246-256.
DeLorenzo RJ. Comparison of status epilepticus with prolonged seizure episodes lasting from 10 to 29 minutes. Epilepsia. 1999 Feb;40(2):164-9.
LaRoche SM, Helmers SL. The New Antiepileptic Drugs: Scientific Review. JAMA. 2004;291:605-614.
Minns AB, Ghafouri N, Clark RF. Isoniazid-induced status epilepticus in a pediatric patient after inadequate pyridoxine therapy. Pediatr Emerg Care. 2010; 26(5):380-1.
Ogilvy CS et al. Recommendations for the Management of Intracranial Arteriovenous Malformations: A Statement for Healthcare Professionals From a Special Writing Group of the Stroke Council, American Stroke Council. Stroke. 2001; 32: 1458-1471
Rosati A et al. Efficacy and safety of ketamine in refractory status epilepticus in children. Neurology. 2012; 79:2355-2358.
Schwartz ID. Hyponatremic seizure in a child using desmopressin for nocturnal enuresis. Arch Pediatr Adolesc Med. 1998 Oct;152(10):1037-8
Trommer BL, Pasternak JF. NMDA receptor antagonists inhibit kindling epileptogenesis and seizure expression in developing rats. Brain Res Dev Brain Res. 1990 May 1;53(2):248-52.
Waterhouse EJ et al. Prospective population-based study of intermittent and continuous convulsive status epilepticus in Richmond, Virginia. Epilepsia. 1999 Jun;40(6).
You have all of the skills you need to care for an acutely ill infant. Learn a few pearls to make this a smoother endeavor.
The Pediatric Assessment Triangle is a rapid, global assessment tool using only visual and auditory clues to make determinations on three key domains: appearance, work of breathing, and circulation to the skin.
The combination of abnormalities determines the category of pathophysiology: respiratory distress, respiratory failure, CNS or metabolic problem, shock, or cardiopulmonary failure.
Appearance
"TICLS"
Tone - the newborn should have a normal flexed tone; the 6 month old baby who sits up and controls her head; the toddler cruises around the room.
Interactiveness - Does the 2 month old have a social smile? Is the toddler interested in what is going on in the room?
Consolability - A child who cannot be consoled at some point by his mother is experiencing a medical emergency until proven otherwise.
Look/gaze - Does the child track or fix his gaze on you, or is there the "1000-yard stare"?
Speech/cry - A vigorously crying baby can be a good sign, when consolable - when the cry is high-pitched, blood-curling, or even a soft whimper, something is wrong.
If the child fails any of the TICLS, then his appearance is abnormal.
Work of Breathing
Children are respiratory creatures - they are hypermetabolic - we need to key in on any respiratory embarrassment.
Look for nasal flaring. Uncover the chest and abdomen and look for retractions. Listen - even without a stethoscope - for abnormal airway sounds like grunting or stridor. Grunting is the child's last-ditch effort to produce auto-PEEP. Stridor is a sign of critical upper airway narrowing.
Look for abnormal positioning, like tripodding, or head bobbing
Circulation to the skin
Infants and children are vasospastic - they can change their vascular tone quickly, depending on their volume status or environment. Without even having to touch the child, you can see signs of pallor, cyanosis, or mottling. If any of these is present, this is an abnormal circulation to the skin.
Pattern of Abnormal Arms = Category of Pathophysiology
Differential Diagnosis in a Sick Infant: "THE MISFITS"
Trauma - birth trauma, non-accidental - check for a cephalohematoma which does not cross suture lines and feels like a ballotable balloon, as well as for subgaleal hemorrhage, which is just an amorphous bogginess that represents a dangerous bleed. Do a total body check.
Heart disease or Hypovolemia - is there a history of congenital heart disease? Was there any prenatal care or ultrasound done? Does this child look volume depleted?
Endocrine Emergencies - Could this be congenital adrenal hyperplasia with low sodium, high potassium, and shock? Look for clitoromegaly in girls, or hyperpigmented scrotum in boys. Could this be congenital hypothyroidism with poor tone and poor feeding? Any history of maternal illness or medications? Congenital hyperthyroidism with high output failure?
Metabolic - What electrolyte abnormality could be causing this presentation? Perhaps diGeorge syndrome with hypocalcemia and seizures?
Inborn Errors of Metabolism - there are over 200 inborn errors of metabolism, but only four common metabolic pathways that cause a child to be critically ill. Searching for an inborn error of metabolism is like looking for A UFO - amino acids, uric acids, fatty acids, organic acids. If the child's ammonia, glucose, ketones, and lactate are all normal in the ED, then his presentation to the ED should not be explained by a decompensation of an inborn error of metabolism.
Seizures - Neonatal seizures can be notoriously subtle - look for little repetitive movements of the arms, called "boxing" or of the legs, called "bicycling"
Formula problems - Hard times sometimes prompt parents to dilute formula, causing a dangerous hyponatremia, altered mental status, and seizures. Conversely, concentrated formula can cause hypovolemia
Intestinal disasters - 10% of necrotizing enterocolitis occurs in full-term babies - look for pneumatosis intestinalis on abdominal XR; also think about aganglionic colon or Hirschprung disease; 80% of cases of volvulus occur within the 1st month of life
Toxins - was there some maternal medication or ingestion? Is there some home remedy or medication used on the baby? Check a glucose ad drug screen
Sepsis - Saved for last - You'll almost always treat the sick neonate empirically for sepsis - think of congenital and acquired etiologies.
Hyperoxia Test
The hyperoxia test is the single most important initial test in suspected congenital heart disease - we can test the child's circulation by his reaction to oxygen on an arterial blood gas. Place the child on a non-rebreather mask, and after several minutes, perform an ABG. (Ideally you obtain a preductal ABG in the right upper extremity, and compare that with one on the lower extremity, but this may not be practical.)
In a normal circulatory system, the pO2 should be high - in the hundreds - and certainly over 250 torr. This effectively excludes congenital heart disease as a factor. If the pO2 on supplemental oxygen is less than 100, then this is extremely predictive of hemodynamically significant congenital heart disease. Between 100 and 250, you have to make a judgement call, and I would side on worst first.
If you are giving this child 100% O2, and he doesn't improve 100% -- that is, his ABG is not at least 100 - then he has congenital heart disease until proven otherwise.
Give prostaglandin if the patient is less than 4 weeks old (typical presentation is within the first 1-2 weeks of life). Start at 0.05 mcg/kg/min. PGE keep the systemic circulation supplied with some mixed venous blood until either surgery or palliation is decided.
Summary Points
* When you see a sick infant, keep THE MISFITS around to keep you out of trouble.
* Before you decide on sepsis, ask yourself, could this be a cardiac problem?
* When in doubt, perform the hyperoxia test.
* All the rest, you have time to look up.
Before You Go: The Availability Heuristic
Selected References
Brousseau T, Sharieff GQ. Newborn Emergencies: The First 30 Days of Life. Pediatr Clin N Am. 2006; 53:69-84.
Cloherty JP, Eichenwald EC, Stark AR: Manual of Neonatal Care, 5th edition. Philadelphia, PA, Lipincott Williams & Wilkins, 2004.
Horeczko T, Young K: Congenital Heart Disease, in Pediatric Emergency Medicine-A Comprehensive Study Guide, 4th Ed. ACEP/McGraw-Hill, 2013.
McGowan et al. Part 15: Neonatal Resuscitation: 2010 American Heart Association Guidelines. Circulation. 2010;122:S909-S919.
Okada PJ, Hicks B. Neonatal Surgical Emergencies. Clin Ped Emerg Med. 2002; 3:3-13.