Categories
Myocardial infarction (MI) in children is uncommon, but underdiagnosed. This is due to two main factors: the etiologies are varied; and the presenting symptoms are “atypical”.
We need a mental metal detector!
Case examples
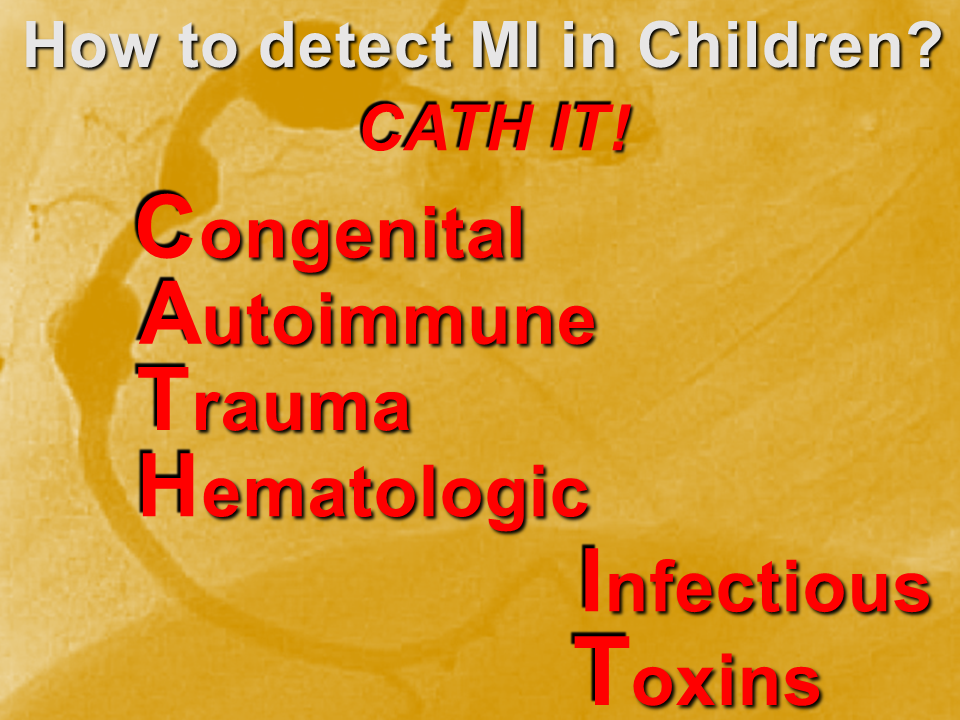
Congenital
Two main presentations of MI due to congenital lesions: novel and known. The novel presentation is at risk for underdiagnosis, due to its uncommonness and vague, atypical symptoms. There are usually some red flags with a careful H&P. The known presentation is a child with a history of congenital heart disease, addressed by corrective or palliative surgery. This child is at risk for expected complications, as well as overdiagnosis and iatrogenia. Risk stratify, collaborate with specialists.
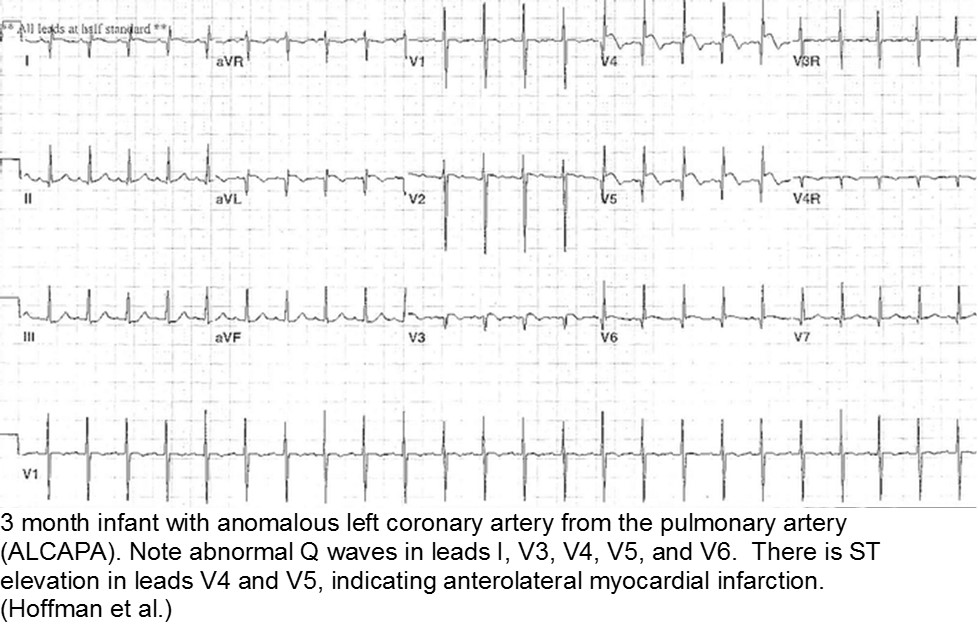
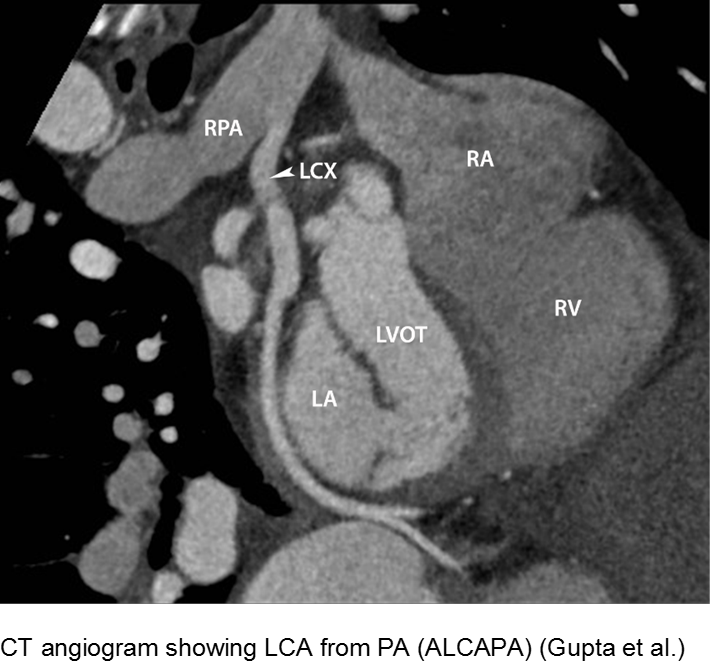
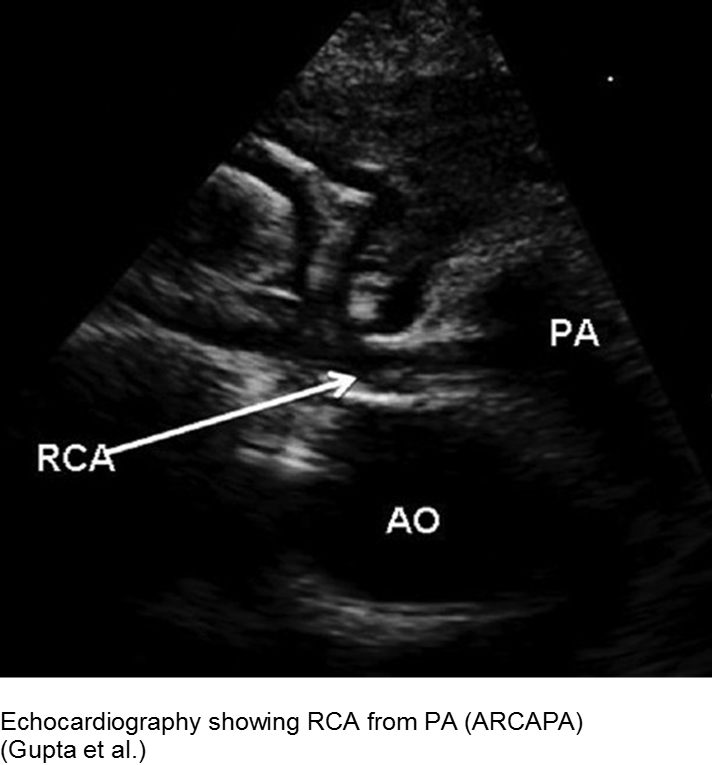
The fussy, sweaty feeder: ALCAPA
Anomalous Left Coronary Artery from the Pulmonary Artery (ALCAPA) is an example of what can go wrong during fetal development: any abnormality in the number, origin, course, or morphology of the coronary arteries can present as a neonate with sweating during feeds (steal syndrome), an infant in CHF, or an older child with failure to thrive or poor exercise tolerance.
The stable child with chest pain: myocardial bridge
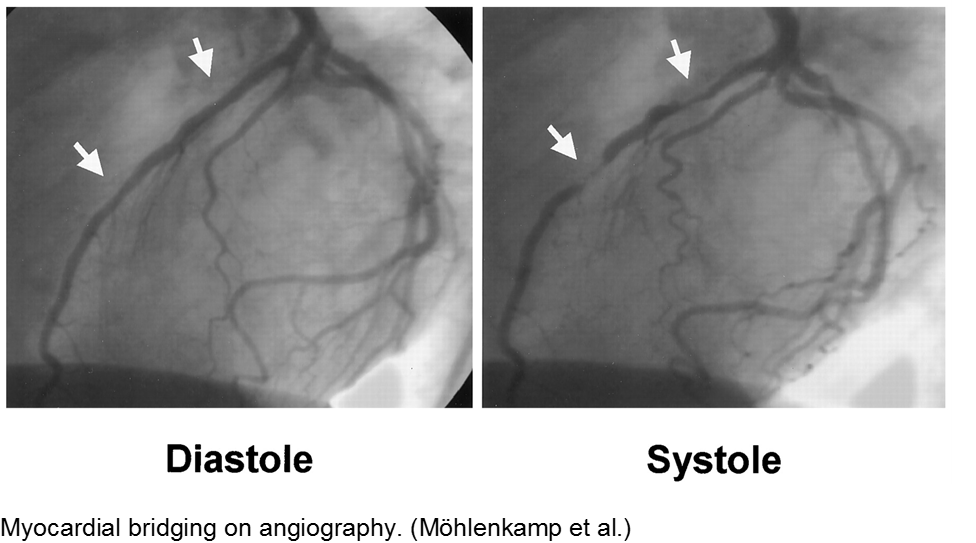
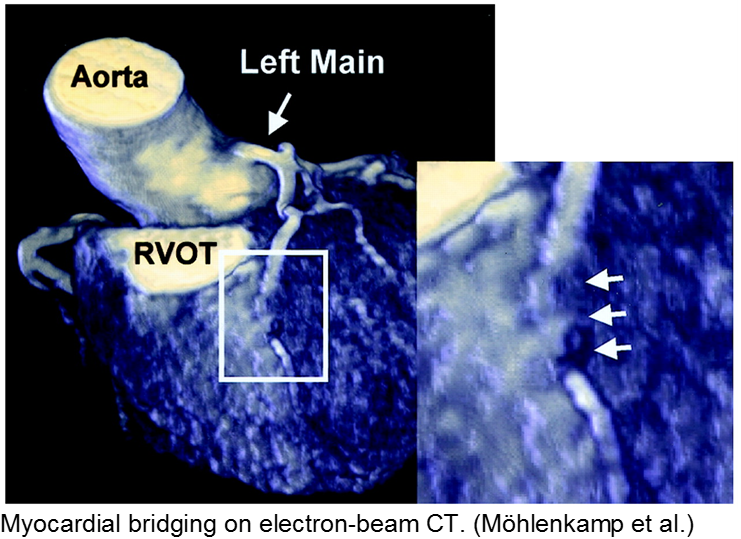
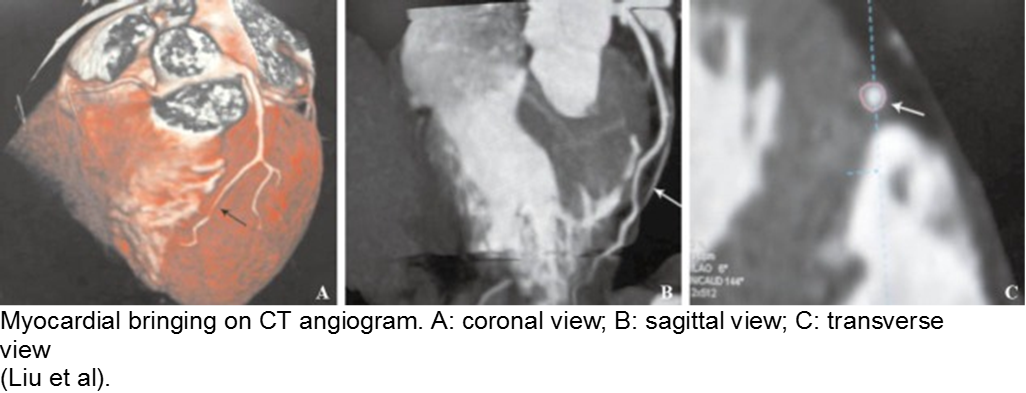
Normal coronary arteries run along the epicardial surface of the heart, with projections into the myocardium. If part of the artery’s course runs within the myocardium (i.e. the artery weaves into and/or out of the myocardium), then there is a myocardial bridge of the coronary artery. With every systolic contraction, the artery is occluded.
Although a myocardial bridge may not cause symptoms (especially at distal portions), the area it supplies is at risk.
With any minor trauma or exertion, demand may outpace supply, resulting in ischemia.
Diagnosis is made on coronary angiography.
The unwell child post-cardiac surgery: Fontan problems
The child with single ventricle physiology may have a Norwood procedure at birth (creation of a neoaorta, atrial septectomy, and Blalock-Taussig shunt), a Bidirectional Glenn procedure at 3-6 months (shunt removed, superior vena cava connected to pulmonary arteries), and a Fontan procedure at about 2-3 years of age (inferior vena cava blood flow is shunted into the pulmonary arteries).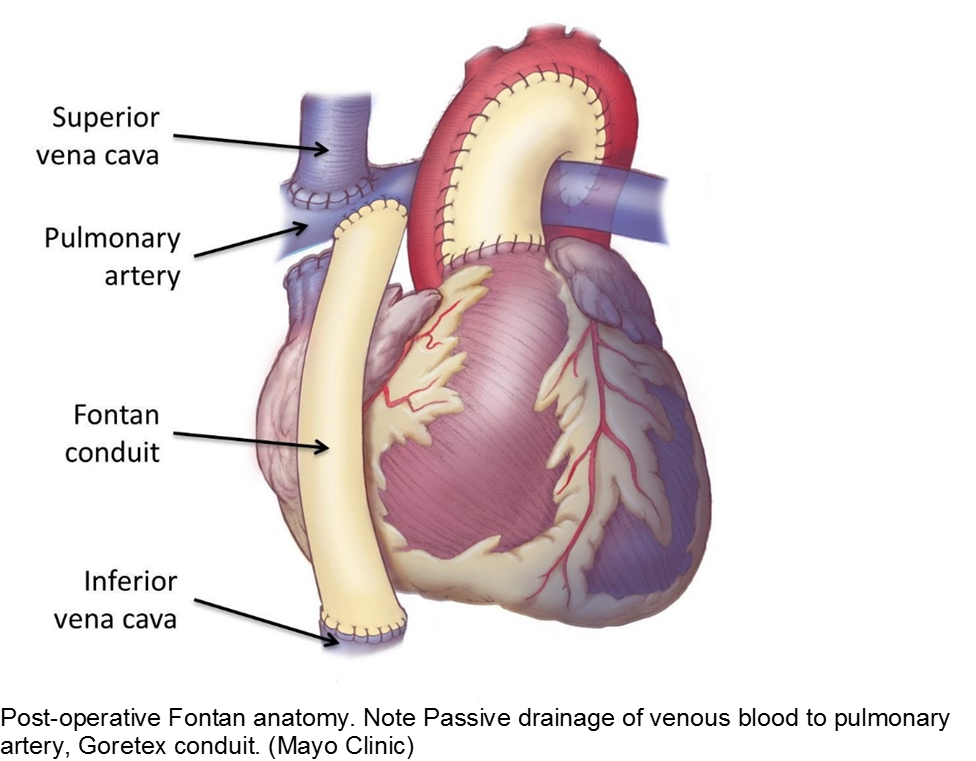
These children depend on their preload to run blood passively into the pulmonary circuit; afterload reduction is also important to compensate for a poor left ejection fraction, as well as to avoid the development of pulmonary hypertension. They are typically on an anticoagulant (often aspirin), a diuretic (e.g. furosemide), and an afterload reduction agent (e.g. enalapril).
Any disturbance in volume status (hyper- or hypovolemia), anticoagulation, or afterload may cause myocardial strain or infarction. Take the child s/p Fontan seriously and involve his specialists early with any concerns.
Autoimmune
The body’s inflammatory-mediated reaction to a real or perceived insult can cause short- and long-term cardiac sequelae. Find out how well the underlying disease is controlled, and what complications the child has had in the past.
The red, hot, crispy, flaky child: acute Kawasaki disease
Kawasaki disease (KD) is an acute systemic vasculitis, diagnosed by the presence of fever for five or more days accompanied by four or more criteria: bilateral conjunctival injection, mucositis, cervical lymphadenopathy, polymorphous rash, and palmar or sole desquamation. The criteria may occur (and disappear) at any time during the illness.
Infants are under double jeopardy with Kawasaki Disease. They are more likely to have incomplete KD (i.e. not fulfill strict criteria) and if they have KD, they are more likely to suffer the dangerous consequences of aneurysm formation (chiefly coronary arteries, but also brain, kidney). Have a low threshold for investigation.
Treatment includes 2 g/kg/day IVIG and high-dose aspirin (30-50 mg/kg/day) acutely, then low-dose aspirin (5 mg/kg/day) for weeks to months. Regular and long-term follow-up with Cardiology is required.
The aftermath: sequelae of Kawasaki disease
The family and child with a history of KD may have psychological trauma and continuous anxiety about the child’s risk of MI. Approximately 4.7% of children who were promptly diagnosed and correctly treated will go on to have cardiac sequelae.
Children who have no detected cardiac sequelae by 8 weeks, typically continue to be asymptomatic up to 20 years later.
Smaller aneurysms tend to regress over time, especially those < 6 mm.
Thrombi may calcify, or the lumen may become stenotic due to myofibroblast proliferation. Children with any coronary artery dilatation from KD should be followed indefinitely.
Giant aneurysms (≥8 mm) connote the highest risk for MI.
Parents often are concerned about recurrence, and any subsequent fever can be distressing. There is a low rate of recurrence for KD: approximately 2%. Infants who have coronary aneurysms are at the highest risk for recurrence.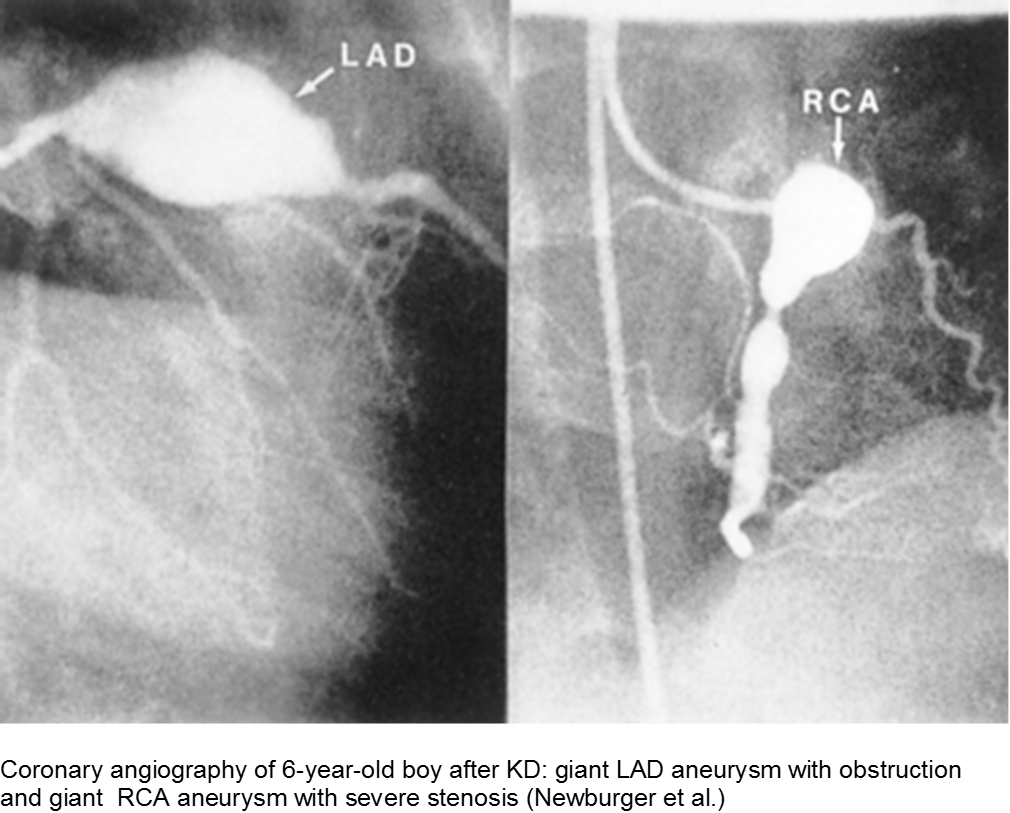
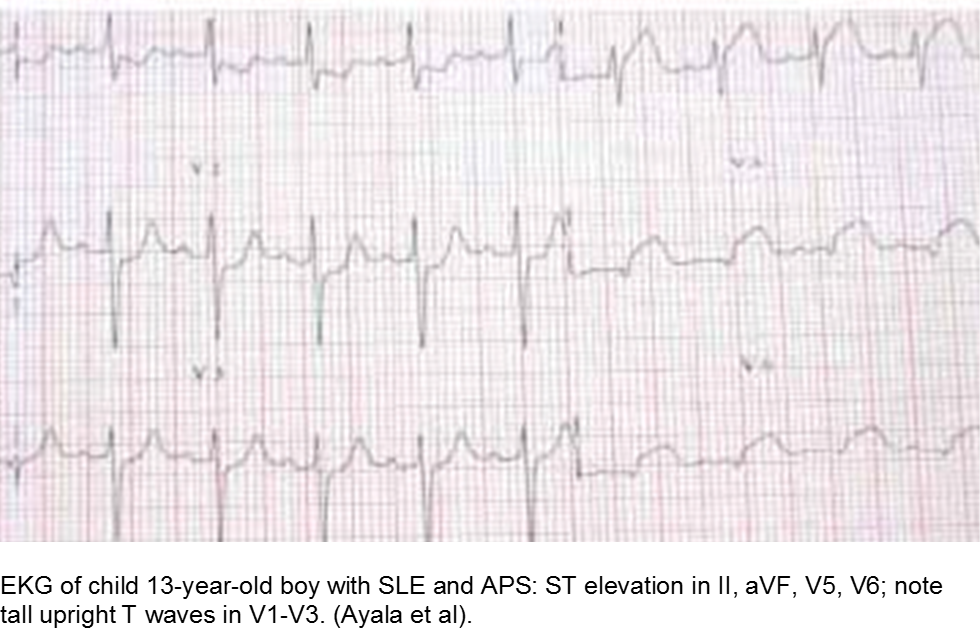
The older child with vague chest complaints and hypercoagulability: Systemic Lupus Erythematosus and Anti-Phospholipid Syndrome
Up to 15% of cases of SLE begin in childhood. Adult criteria are used, with the caveat that the diagnosis of SLE in children can be challenging; many children only manifest a few of the criteria initially before going on to develop further systemic involvement.
The Systemic Lupus International Collaborating Clinics (SLICC) revised the criteria in 2012. The patient should have ≥4/17 clinical and/or immunologic criteria. The clinical criteria are: acute cutaneous (malar); chronic cutaneous (discoid); oral; alopecia; synovitis; serositis; renal; neurologic; hemolytic anemia; leukopenia; or thrombocytopenia. The immunologic criteria are: ANA; anti-dsDNA; anti-Sm; antiphospholipid; low complement; and/or Direct Coombs (in absence of hemolytic anemia). At least one criterion should be clinical, and at least one should be immunologic.
Children with antiphospholipid syndrome (APS) may occur with or without SLE. Patients are at risk for venous and arterial thrombi formation. APS may also cause structural damage, such as valvular thickening and valvular nodes (Libman-Sacks endocarditis). Mitral and aortic valves are at the highest risk.
Although most children with chest pain will not have MI, those with comorbidities should be investigated carefully.
Trauma
Direct, blunt trauma to the chest can cause myocardial stunning, dysrhythmias, or an asymptomatic rise in Troponin I. However, some children are at risk for disproportionate harm due to a previously unknown risk factor. Clinically significant cardiac injury occurs in up to 20% of patients with non-penetrating thoracic trauma.
The motor vehicle collision: blunt myocardial injury
Direct trauma (steering wheel, airbag, seatbelt), especially in fast acceleration-deceleration injury, may cause compression of the heart between the sternum and the thoracic spine.
Electrocardiography (ECG) should be performed on any patient with significant blunt chest injury. A negative ECG is highly consistent with no significant blunt myocardial injury.
Any patient with a new abnormality on ECG (dysrhythmia, heart block, or signs of ischemia) should be admitted for continuous ECG monitoring.
Elevation in troponin is common, but not predicted. A solitary elevated troponin without ECG abnormality is of unclear significance. Author’s advice: obtain troponin testing if there is an abnormal ECG, more than fleeting suspicion of BCI, and/or the child will be admitted for monitoring.
Hemodynamically labile children should be resuscitated and a stat transesophageal echocardiogram obtained.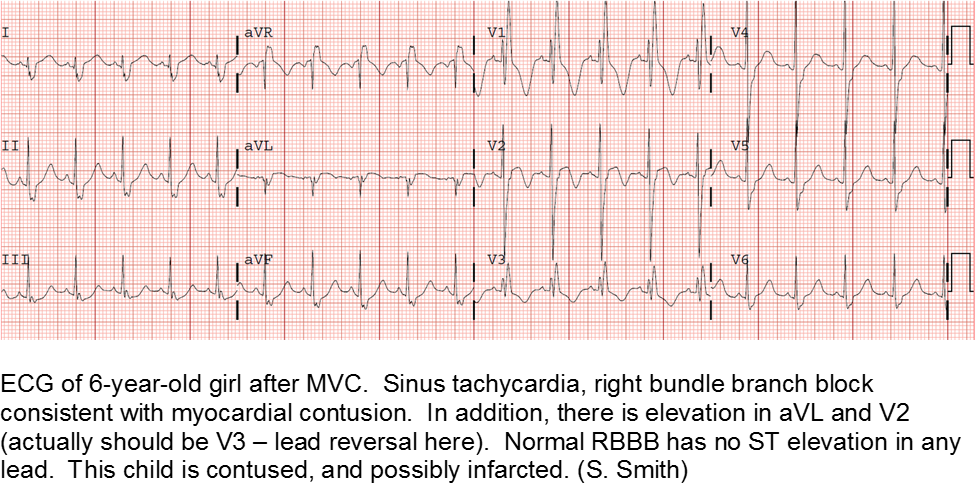
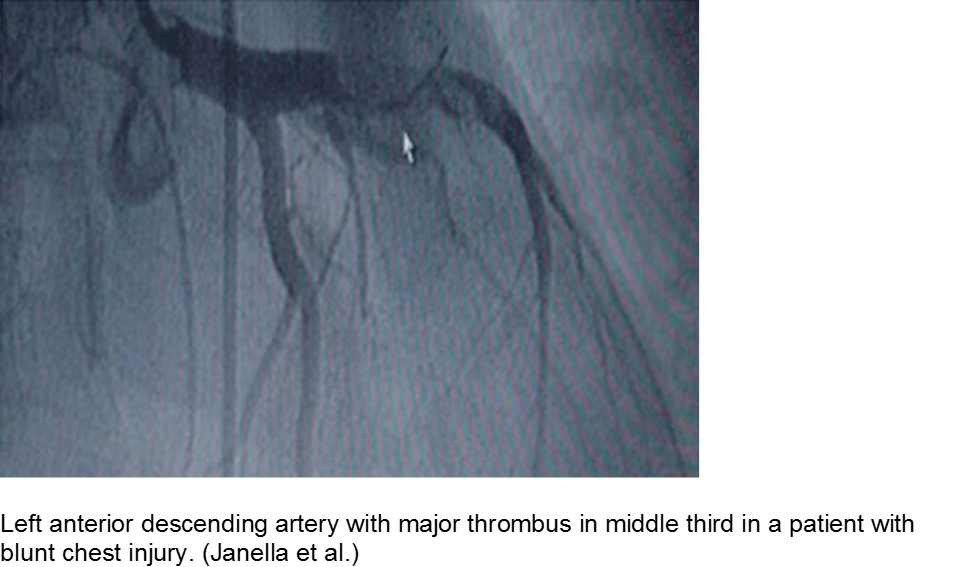
The high-velocity object: coronary artery dissection or thrombus
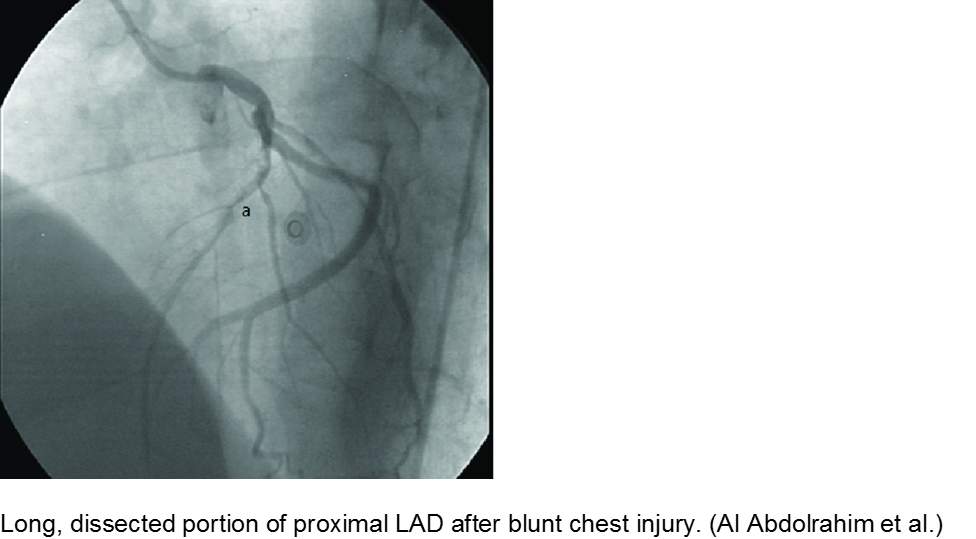
Direct trauma (e.g. MVC, baseball, high-velocity soccer ball) may cause damage to the left anterior descending artery or left circumflex artery, at the highest risk due to their proximity to the chest wall. Thrombosis and/or dissection may result, often presenting in a focal pattern of ischemia on the ECG.
Echocardiography may reveal valvular damage related to the injury, as well as effusion and ejection fraction. Since there is often a need to investigate the coronary anatomy, percutaneous coronary intervention (PCI) is recommended.
The minor trauma with disproportionate complaint: myocardial bridge
As mentioned in the congenital section (above), a known variation of a coronary artery’s course involves weaving in and out of the myocardium, creating a baseline risk for ischemia. Even minor trauma in a child with a myocardial bridge may cause acute thrombus, or slow stenosis from resulting edema. Unfortunately, the presence of myocardial bridging is often unknown at the time of injury. Approximately 25% of the population may have myocardial bridging, based on autopsy studies. Take the child seriously who has disproportionate symptoms to what should be a minor injury.
Hematologic
Coagulopathic and thrombophilic states may predispose children to focal cardiac ischemia. The best documented cormorbidity is sickle cell disease, although other pro-thrombotic conditions also put the child at risk.
The child with sickle cell disease and chest pain: when it’s not acute chest syndrome
Sickle cell disease (SCD) can affect any organ system, although the heart is traditionally considered a lower-risk target organ for direct sickling and ischemia. The major cardiac morbidity in sickle cell is from strain, high-output failure and multiple, serial increases in myocardial demand, causing left ventricular hypertrophy and congestive heart failure.
However, there is mounting evidence that acute myocardial ischemia in sickle cell disease may be underappreciated and/or attributed to other causes of chest pain.
Other cardiac sequelae from SCD include pulmonary hypertension, left ventricular dysfunction, right ventricular dysfunction, and chronic iron overload.
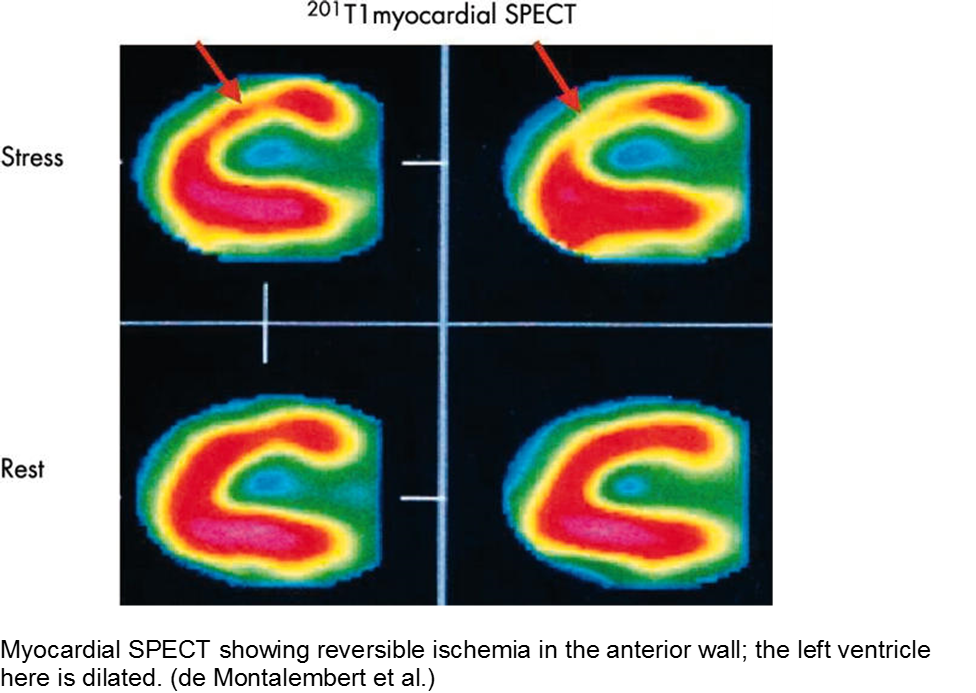
Evidence of myocardial ischemia/infarction in children with SCD has been demonstrated on single-photon emission computed tomography (SPECT) scan.
The puffy faced child with chest pain: nephrotic syndrome hypercoagulability
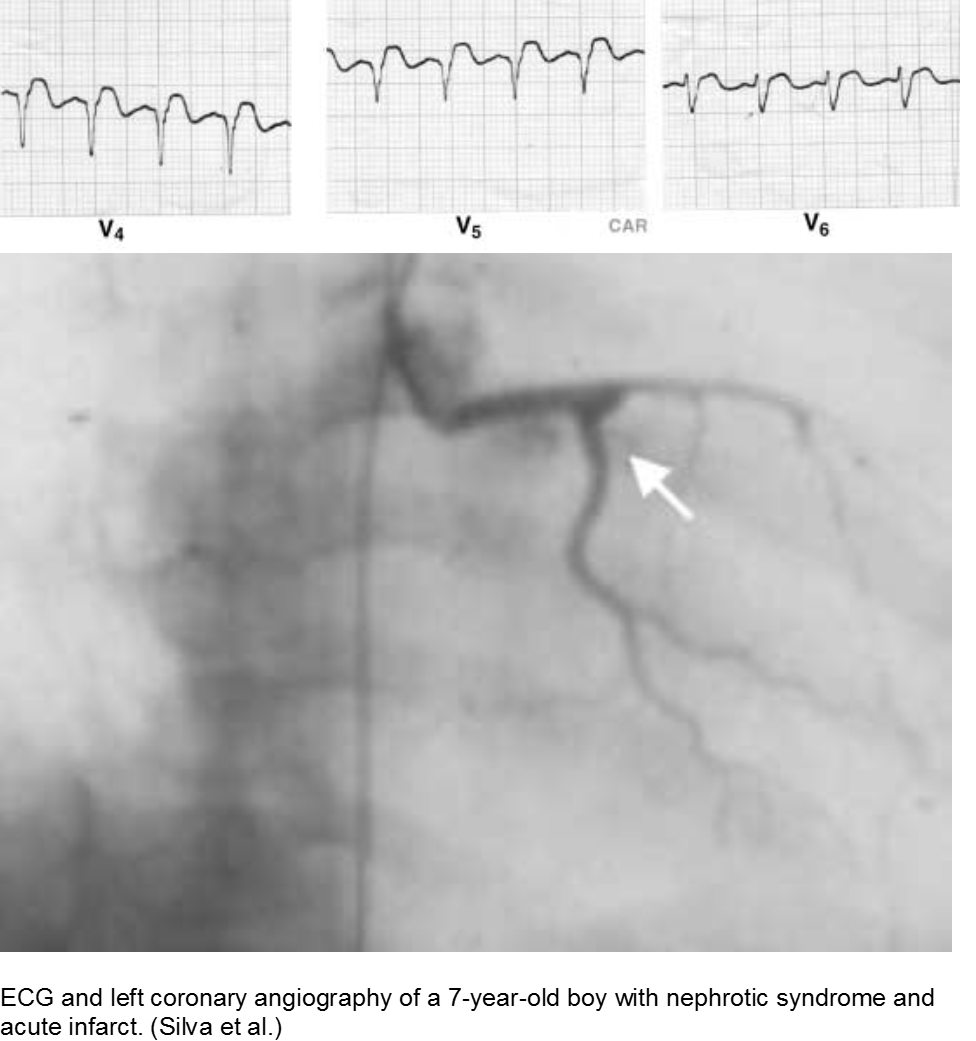
Children who suffer from nephrotic syndrome lose proteins that contribute to the coagulation cascade. In addition, lipoprotein profiles are altered: there is a rise in the very low-density lipoproteins (LDL), contributing to accelerated atherosclerosis. Typically nephrotic patients have normal levels of high-density lipoproteins (HDL), unless there is profuse proteinuria.
Children with difficult-to-control nephrotic syndrome (typically steroid-resistant) may form accelerated plaques that rupture, causing focal MI, as early as school age.
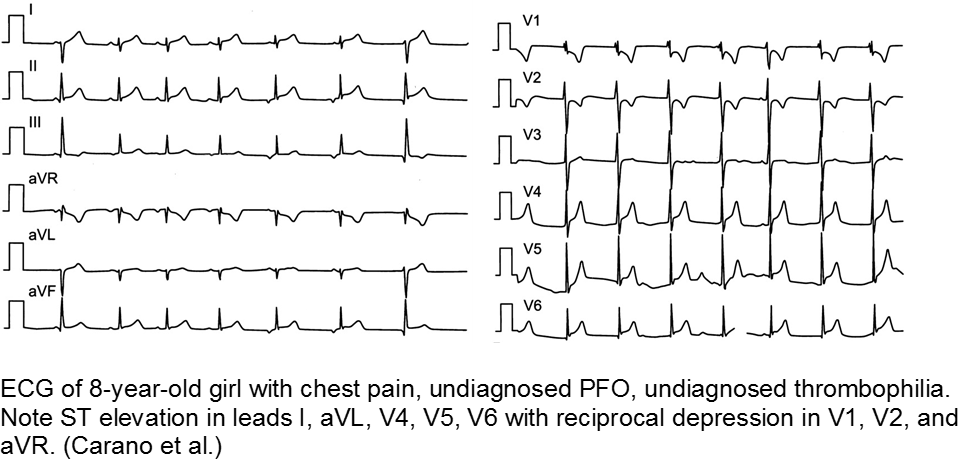
The previously well child now decompensated: undiagnosed thrombophilia
Asymptomatic patent foramen ovale (PFO) is the cause of some cases of cryptogenic vascular disease, such as stroke and MI. However, the presence of PFO alone does not connote higher risk. When paired with an inherited or acquired thrombogenic condition, the venous thrombus may travel from the right-sided circulation to the left, causing distal ischemia. Many of these cases are unknown until a complication arises.
The chronically worried, now with a reason: hypercholesterolemia
A family history of adult-onset hypercholesterolemia is not necessarily a risk factor for early complications in children, provided the child does not have the same acquired risk factors as adults (e.g. obesity, sedentary lifestyle, smoking, etc). Parents may seek help in the ED for children with chest pain and no risk factors, but adult parents who have poor cholesterol profiles.
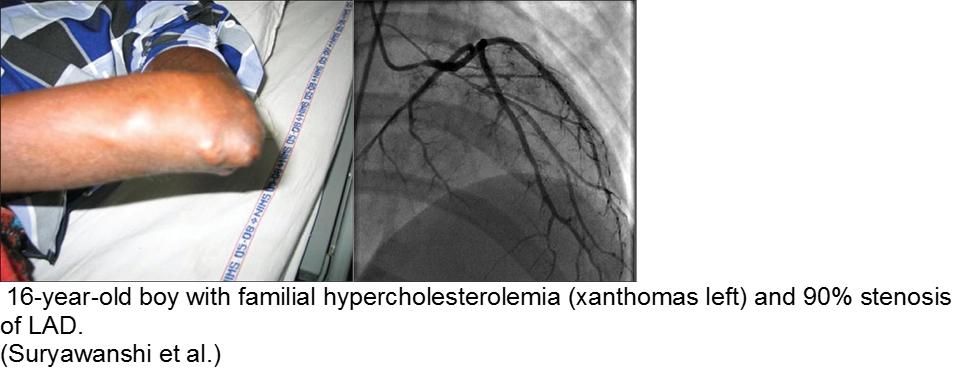
The exception is the child with familial hypercholesterolemia, who is at risk for accelerated atherosclerosis and MI.
Infectious
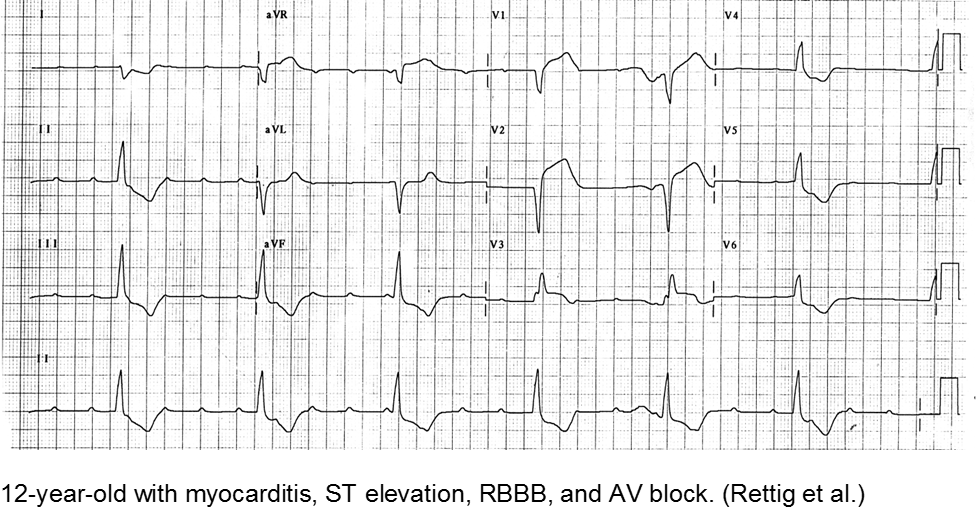
Myocarditis has varied etiologies, including infectious, medications (chemotherapy agents), immunologic (rheumatologic, transplant rejection), toxins (arsenic, carbon monoxide, heavy metals such as iron or copper), or physical stress (electrical injury, heat illness, radiation).
In children, the most common cause of myocarditis is infectious (viruses, protozoa, bacteria, fungal, parasites). Of these, viral causes are the most common (adenovirus, enterovirus, echovirus, rubella, HHV6).
The verbal child may complain of typical chest complaints, or may come in with flu-like illness and tachycardia or ill appearance out of proportion to presumed viral illness.
The most common presenting features in children with myocarditis are: shortness of breath, vomiting, poor feeding, hepatomegaly, respiratory distress, and fever.
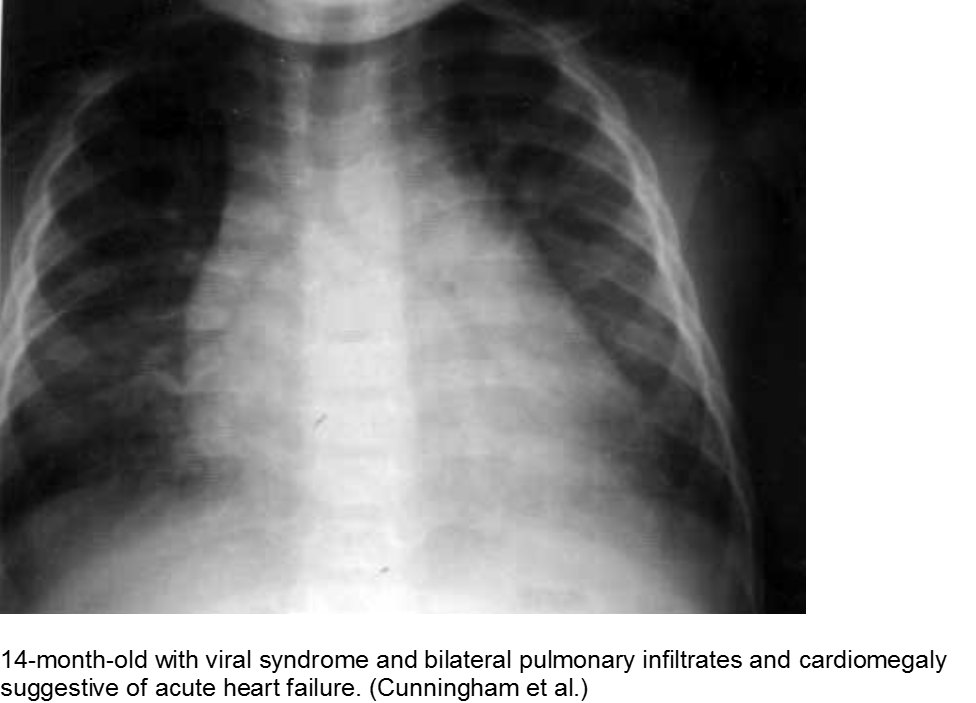
The infant in shock after a ‘cold’: myocarditis
Beware of the poor feeding, tachycardic, ill appearing infant who “has a cold” because everyone else around him has a ‘cold’. That may very well be true, but any virus can be invasive with myocardial involvement. Infants are only able to increase their cardiac output through increasing their heart rate; they cannot respond to increased demands through ionotropy. Look for signs of acute heart failure, such as hepatomegaly, respiratory distress, and sacral edema.
The child with tachycardia out of proportion to complaint: myocarditis
The previously healthy child with “a bad flu” may simply be very symptomatic from influenza-like illness, or he may be developing myocarditis. Look for chest pain and tachycardia out of proportion to presumed illness, and constant chest pain, not just associated with cough.
The “pneumonia” with suspicious chest x-ray: myocarditis
Acute heart failure may mimic viral pneumonia. Look for disproportionate signs and symptoms.
Toxins
Younger children may get into others’ medications, be given dangerous home remedies, take drugs recreationally, have environmental exposures (heavy metals), suffer from a consequence of a comorbidity (iron or copper overload) or have adverse events from generally safe medications.
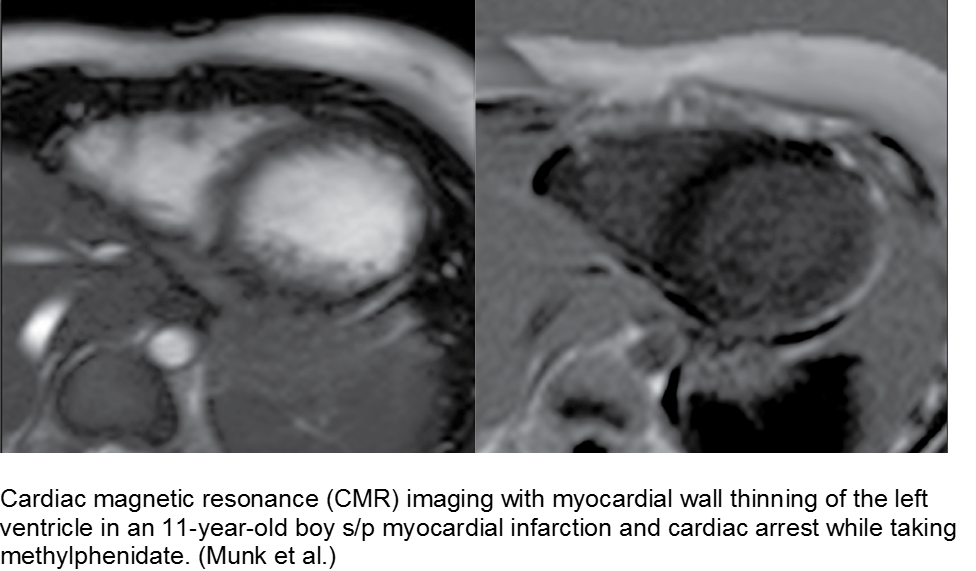
The hyperactive boy with a hyperactive precordium: methylphenidate
Attention deficit hyperactivity disorder (ADHD) is growing in rate of diagnosis and use of medications. As the only medical diagnosis based on self-reported criteria, many children are given stimulants regardless of actual neurologic disorder; with a higher proportion of children exposed to stimulants, adverse effects are seen more commonly.
Methylphenidate is related to amphetamine, and they both are dopaminergic drugs. Their mechanisms of action are different, however. Methylphenidate increases neuronal firing rate. Methamphetamine reduces neuronal firing rate; cardiovascular sequelae such as MI and CHF are more common in chronic methamphetamine use.
Although methylphenidate is typically well tolerated, risks include dysrhythmias such as ventricular tachycardia.
The child with seizure disorder and chest pain: anti-epileptics
Some anti-epileptic agents, such as carbamazepine, promote a poor lipid profile, leading to atherosclerosis and early MI. Case reports include school-aged children on carbamazepine who have foamy cells in the coronary arteries, aorta, and vasa vasorum on autopsy. It is unclear whether this is a strong association.
The spice trader: synthetic cannabinoids
Synthetic cannabinoids are notoriously difficult to regulate and study, as the manufacturers label them as “not for human consumption”. Once reports surface of abuse of a certain compound, the formula is altered slightly and repackaged, often in a colorful or mysterious way that is attractive to teenagers.
The misperceptions are: are a) synthetics are related to marijuana and therefore safe and b) marijuana is inherently “safe”. Both tend to steer unwitting teens to take these unknown entities. Some suffer MI as a result.
Exposure to tetrahydrocannabinol (THC) in high-potency marijuana has been linked to myocardial ischemia, ventricular tachycardia, and ventricular fibrillation. Marijuana can increase the heart rate from 20-100%, depending on the amount ingested.
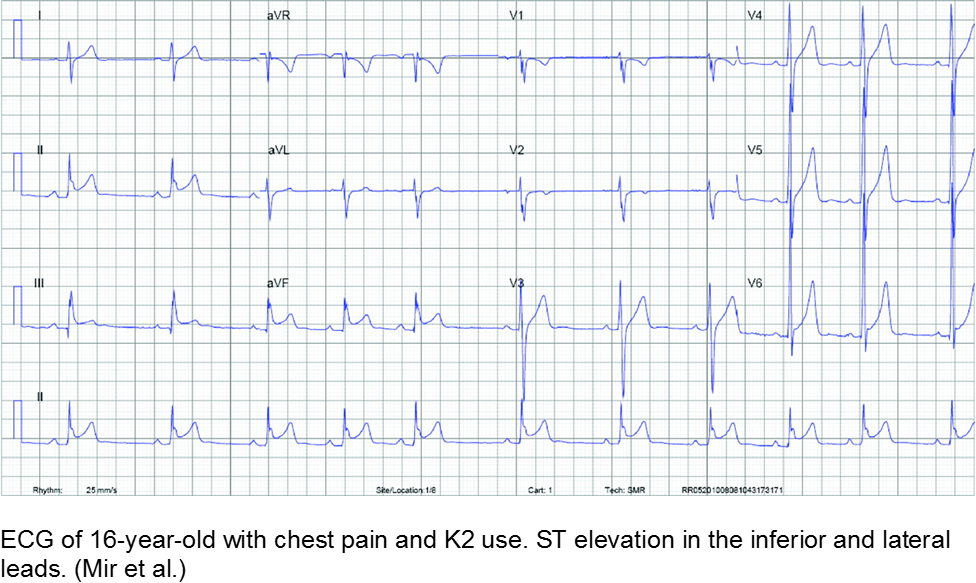
K2 (“kush 2.0”) or Spice (Zohai, Genie, K3, Bliss, Nice, Black Mamba, fake weed, etc) is a mixture of plant leaves doused in synthetic chemicals, including cannabinoids and fertilizer (JWH-108), none of which are tested or safe for human consumption.
Synthetic cannabinoids have a higher affinity to cannabinoid receptors, conferring higher potency, and therefore worse adverse effects. They are thought to be 100 to 800 times more potent as marijuana.
Bath salts (Purple Wave, Zoom, Cloud Nine, etc) can be ingested, snorted, or injected. They typically include some form of cathinone, such as mephedrone, similar to the substance found in the naturally occurring khat plant. Hallucinations, palpitations, tachycardia, MI, and dysrhythmias have been reported from their use as a recreational drug.
Chest pain with marijuana, synthetic cannabinoid, or bath salt ingestion should be investigated and/or monitored.
Riding that train: high on cocaine
Cocaine is a well-known cause of acute MI in young people. In addition to the direct stimulant causes acutely, such as hypertension, tachycardia, and impaired judgement (coingestions, risky behavior), chronic cocaine use has long-term sequelae. Cocaine causes accelerated atherosclerosis. That, in conjunction with arterial vasospasm and platelet activation, is a recipe for acute MI in the young.
Cranky: methamphetamine
Methamphetamine is a highly addictive stimulant that is relatively inexpensive and widely available. Repeated use causes multiple psychiatric, personality, and neurologic changes. Risky behavior, violence, and motor vehicle accidents are all linked to this drug.
Like cocaine, methamphetamine may cause fatal dysrhythmias, acute MI from demand ischemia, and long-term sequelae such as congestive heart failure.
Summary
Acute MI is a challenging presentation in children:
- Easily missed: uncommon and atypical
- Varied etiology
- Respect vague symptoms with a non-reassuring H&P
- Try to detect it: CATH IT!
References
Congenital
AboulHosn JA et al. Fontan Operation and the Single Ventricle. Congenit Heart Dis. 2007; 2:2-11.
Aliku TO et al. A case of anomalous origin of the left coronary artery presenting with acute myocardial infarction and cardiovascular collapse. African Health Sci. 2014; 14(1): 23-227.
Andrews RE et al. Acute myocardial infarction as a cause of death in palliated hypoplastic left heart syndrome. Heart. 2004; 90:e17.
Canale LS et al. Surgical treatment of anomalous coronary artery arising from the pulmonary artery. Interactive Cardiovascaulr and Thoracic Surgery. 2009; 8:67-69.
Güvenç O et al. Correctable Cause of Dilated Cardiomyopathy in an Infant with Heart Failure: ALCAPA Syndrome. J Curr Pediatr. 2017; 15:47-50.
Hastings RS et al. Embolic Myocardial Infarction in a Patient with a Fontan Circulation. World Journal for Pediatric Congenital Heart Surgery. 2014; 5(4)L631-634.
Hoffman JIE et al. Electrocardiogram of Anomalous Left Coronary Artery From the Pulmonary Artery in Infants. Pediatr Cardiol. 2013; 34(3):489-491.
Kei et al. Rare Case of Myocardial Infarction in a 19-Year-Old Caused by a Paradoxical Coronary Artery Embolism. Perm J.2015; 19(2):e107-e109.
Liu Y, Miller BW. ALCAPA Presents in an Adult with Exercise Inlerance but Preserved Cardiac Function. Case Reports Cardiol. 2012; AID 471759.
Möhlenkamp S et al. Update on Myocardial Bridging.Circulation. 2002;106:2616-2622.
Murgan SJ et al. Acute myocardial infraction n the neonatal period. Cardiol Young. 2002; 12:411-413.
Sieweke JT et al. Myocardial infarction in grown up patients with congenital heart disease: an emergening high-risk combination. International Journal of Cardiology. 2016; 203:138-140.
Schwerzmann M et al. Anomalous Origin of the Left Coronary Artery From the Main Pulmonary Artery in Adults. Circulation. 2004; 110:e511-e513.
Tomkewicz-Pajak L et al. Arterial stiffness in adult patients after Fontan procedure. Cardiovasculr Ultrasound. 2014; 12:15.
Varghese MJ et al. The caveats in the diagnosis of anomalous origin of left coronary artery from pulmonary artery (ALCAPA). Images Paediatr Cardiol. 2010; 12(3): 3–8.
Autoimmune
Ayala et al. Acute Myocardial Infarction in a Child with Systemic Lupus Erythematosus and Antiphospholipid Syndrome. Turk J Rheumatol. 2009; 24:156-8.
Nakano H et al. Clinical characteristics of myocardial infarction following Kawasaki disease: Report of 11 cases. J Pediatr. 1986; 108(2):198-203.
Pongratz G et al. Myocardial infarction in an adult resulting from coronary aneurysms previously documented in childhood after an acute episode of Kawasaki’s disease. European Heart J. 1994. 15:1002-1004.
Newburger JW et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease. A Statement for Health Professionals From the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747-2771.
Son MB et al. Kawaski Disease. Pediatr Rev. 2013; 34(4).
Yuan S. Cardiac surgical procedures for the coronary sequelae of Kawasaki disease. Libyan J Med. 2012; 7:19796.
Trauma
Abdolrahim SA et al. Acute Myocardial Infarction Following Blunt Chest Trauma and Coronary Artery Dissection. J Clin Diagnost Res. 2016; 10(6):14-15.
Galiuto L et al. Post-traumatic myocardial infarction with hemorrhage and microvascular damage in a child with myocardial bridge: is coronary anatomy actor or bystander. Signa Vitae. 2013; 8(2):61-63.
Janella BL et al. Acute Myocardial Infarction related to Blunt Thoracic Trauma. Arq Bras Cardiol. 2006; 87:e168-e171.
Liu X et al. Acute myocardial infarction in a child with myocardial bridge World J Emerg Med. 2011; 2(1):70-72.
Long WA et al. Childhood Traumatic Infarction Causing Left Ventricular Aneurysm: Diagnosis by Two-Dimensional Echocardiography. JACC. 1985; 5(6):1478-83.
Smith S. Right Bundle Branch Block after Blunt Trauma: A Tragic Case. [Blog Post] July 22, 2012. Retrievable at: http://hqmeded-ecg.blogspot.com/2012/07/right-bundle-branch-block-after-blunt.html.
Hematologic
Carano N et al. Acute Myocardial Infarction in a Child: Possible Pathogenic Role of Patent Foramen Ovale Associated with Heritable Thrombophilia. Pediatr. 2004; 114(2):255-258.
Chacko P et al. Myocardial Infarction in Sickle Cell Disease. J Cardiovascl Transl Res. 2013; 6(5):752-761.
De Montalembert M et al. Myocardial ischaemia in children with sickle cell disease. Arch Dis Child. 2004; 89:359-362.
Gladwin MT et al. Cardiovascular Abnormalities in Sickle Cell Disease. JACC. 2012; 59(13):1123-1133.
Osula S et al. Acute myocardial infarction in young adults: causes and management. Postgrad Med J. 2002; 78:27-30.
Silva JMP et al. Premature acute myocardial infarction in a child with nephrotic syndrome. Pediatr Nephrol. 2002; 17:169-172.
Suryawanshi SP. Myocardial infarction in children: Two interesting cases. Ann Pediatr Cardiol. 2011 Jan-Jun; 4(1): 81–83.
Infectious
Cunningham R et al. Viral myocarditis Presenting with Seizure and Electrocardiographic Findings of Acute Myocardial Infarction in a 14-Month-Old Child. Ann Emerg Med. 2000; 35(6):618-622.
De Vettten L et al. Neonatal Myocardial Infarction or Myocarditis? Pediatr Cardiol. 2011; 32:492-497.
Durani Y et al. Pediatric myocarditis: presenting clinical characteristics. Am J Emerg Med. 2009; 27:942-947.
Erden I et al. Acute myocarditis mimicking acute myocardial infarction associated with pandemic 2009 (H1N1) influenza virus. Cardiol J. 2011; 552-555.
Hover MH et al. Acute Myocarditis Simulating Myocardial Infarction in a Child. Pediatr. 1191; 87(2):250-252.
Lachant D et al. Meningococcemia Presenting as a Myocardial Infarction. Case Reports in Critical Care. 2015; AID 953826.
Laissy JP et al. Differentating Myocardial Infarction from Myocarditis. Radiology. 2005; 237(1):75-82.
Miranda CH et al. Evaluation of Cardiac Involvement During Dengue Viral Infection. CID. 2013; 57:812-819.
Rettig JS et al. Myocarditis in Children Requiring Critical Care Transport. In: "Diagnosis and Treatment of Myocarditis", Milei J, Ambrosio G (Eds). DOI: 10.5772/56177.
Toxins
De Chadarévian JP et al. Epilepsy, Atherosclerosis, Myocardial Infarction, and Carbamazepine. J Child Neurol. 2003; 18(2):150-151.
McIlroy G et al. Acute myocardial infarction, associated with the use of a synthetic adamantly-canabinoid: a case report. BMC Pharmacology and Toxicology. 2016; 17:2.
Mir A et al. Myocardial Infarction Associated with Use of the Synthetic Cannabinoid K2. Pediatr. 2011; 128(6):1-6
Munk K et al. Cardiac Arrest following a Myocardial Infarction in a Child Treated with Methylphenidate. Case Reports Pediatr. 2015; AID 905097.
Rezkalla SH et al. Cocaine-Induced Acte Mycardial Infarction. Clin Med Res. 2007; 5(3):172-176.
Schelleman H et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012 Feb;169(2):178-85.
Sheridan J et al. Injury associated with methamphetamine use: a review of the literature. Harm Reduction Journal, 2006; 3(14):1-18.
Stiefel G et al. Cardiovascular effects of methylphenidate, amphetamines and atomoxetine in the treatment of attention-deficit hyperactivity disorder. Drug Saf. 2010 Oct 1;33(10):821-42.
This post and podcast are dedicated to Edwin Leap, MD for his sanity and humanity in the practice of Emergency Medicine. Thank you, Dr Leap for all that you do.